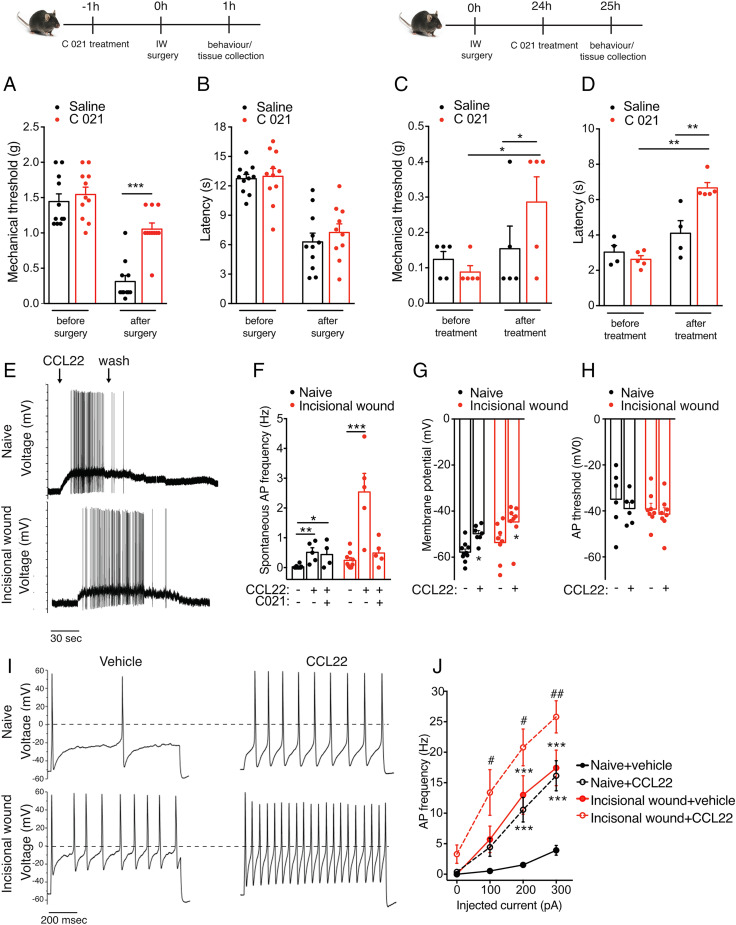
Fig. 4.
CCR4 activation modulates postoperative pain and results in altered membrane properties and increased AP firing. (A and C) Mechanical hypersensitivity was significantly reduced in animals in which CCR4 is inhibited 1 h before incision (n = 10/group). (B and D) Thermal hypersensitivity was significantly reduced in animals treated with C 021 24 h after incision. (E and F) CCL22 induces an increase in AP generation in cultured DRG neurons isolated from both naïve and 24 h postoperative mice, and this effect is partially reversed by pretreatment with CCR4 antagonist (1 µM) (naïve: 0.025 ± 0.01 Hz, 0.51 ± 0.15 Hz after CCL22, and 0.44 ± 0.22 Hz after CCR4 antagonist and CCL22; n = 5, 5, and 4 neurons recorded; incisional wound: 0.24 ± 0.09 Hz, 2.54 ± 0.6 Hz after CCL22, and 0.049 ± 0.18 Hz after CCR4 antagonist and CCL22; n = 9, 5, and 5 neurons recorded). (G and H) The increase in AP frequency is associated with a membrane depolarization (from −57.82 ± 1.35 mV to −49.8 ± 1.38 mV, from −53.63 ± 2.99 mV to −44.68 ± 2.79 mV, and from −55.2 ± 1.72 mV to −46.4 ± 2.1 mV after CCL22 treatment in naïve and incisional wound neurons, respectively; n = 10, 7, 8, 8, 8, and 8). However, the AP threshold remained unchanged. (I and J) CCL22 treatment increased the evoked neuronal activity as shown by these representative data and mean values on the right (n = 7 and 5 for naïve and incisional wound, respectively). The AP discharge was evoked by a 200-pA current injection (1 s) in control (Upper) and 24 h after surgery (Lower). n = 4 mice/group in E–J. Data are represented as mean ± SEM; all data were analyzed using two-way ANOVA with post hoc Bonferroni test (*P < 0.05, **P < 0.01, and ***P < 0.001; # <0.05 and ## <0.01). Data are representative of at least n = 2 experiments.