Table 2.
Hantzsch synthesis of polyhydroquinoline derivatives in the presence of Zn-MOF in PEG-400 at 80 °C.
|
| ||||||
|---|---|---|---|---|---|---|
| Entry | Aryl aldehyde | Product | Time (min) | Yield (%)a,b | Melting point | |
| Measured | Literature | |||||
| 1 |

|
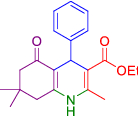
|
150 | 83 | 216–218 | 217–21950 |
| 2 |
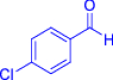
|

|
85 | 96 | 237–240 | 234–23750 |
| 3 |
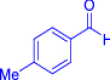
|

|
120 | 74 | 252–255 | 252–25551 |
| 4 |
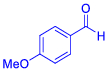
|

|
70 | 58 | 247–250 | 245–24751 |
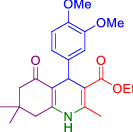
| 5 |

|
|
180 | 69 | 201–204 | 203–20450 |
| 6 |

|

|
150 | 79 | 263–265 | 246–24850 |
| 7 |

|

|
75 | 95 | 173–175 | 176–17850 |
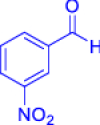
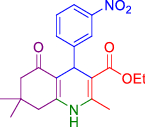
| 8 |
|
|
90 | 85 | 217–219 | 216–21850 |
| 9 |

|

|
90 | 97 | 217–219 | 216–21850 |
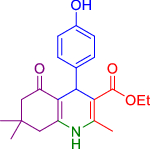
| 10 |

|
|
150 | 92 | 227–229 | 231–23351 |
| 11 |
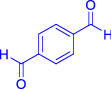
|
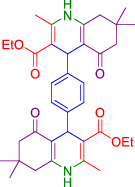
|
90 | 91 | 301–303 | 303–30550 |
aIsolated yields.
bReaction conditions: Aromatic aldehyde (1 mmol), dimedone (1 mmol), ethyl acetoacetate (1 mmol), ammonium acetate (1.2 mmol), Zn-MOF (10 mg) and PEG-400 (2 mL) at 80 °C.
cReaction conditions: Aromatic aldehyde (1 mmol), dimedone (2 mmol), ethyl acetoacetate (2 mmol), ammonium acetate (2.4 mmol), Zn-MOF (20 mg) and PEG-400 ( 4 mL) at 80 °C.
