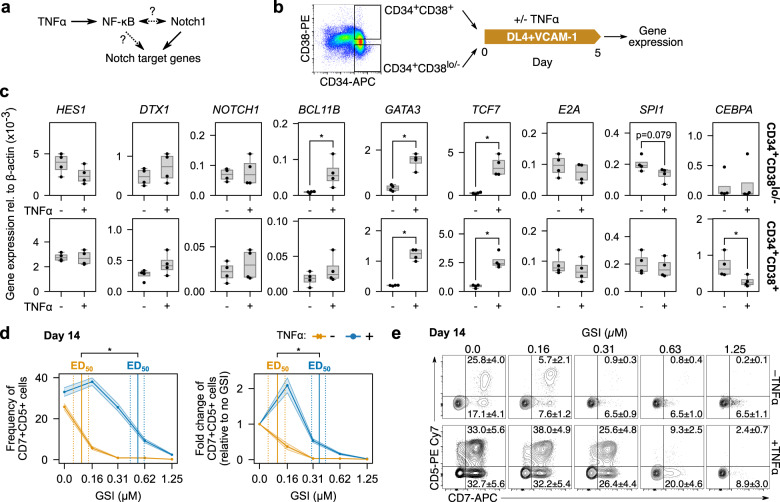
Fig. 2. Synergy between TNFα and the Notch pathway compensates for low Notch activation.
a TNFα activates the NF-κB pathway, which may regulate Notch target genes or regulate Notch itself. b To investigate the ways that TNFα may be interacting with Notch, CD34+ HSPCs were sorted into CD38lo/− and CD38+ fractions and seeded separately on DL4 + VCAM-1 with and without TNFα. Gene expression was measured using qPCR after 5 days of culture. c Both the CD38lo/− and CD38+ fractions upregulated GATA3 and TCF7 in response to TNFα while only CD38lo/− HSPCs upregulated BCL11B. No differences were observed in any other Notch target genes, implying that TNFα is not regulating Notch itself. CD38lo/− but not CD38+ HSPCs downregulated SPI1 slightly in response to TNFα. In contrast, only the CD38+ fraction significantly downregulated CEBPA when cultured with TNFα. Bar plots show a median and interquartile range of n = 4 independent UCB donors. d CD34+ HSPCs were seeded on DL4 + VCAM-1 for 14 days with increasing concentrations of γ-secretase inhibitor (GSI) to inhibit Notch activation. TNFα was able to maintain CD7+CD5+ cell generation with a significantly higher concentration of GSI than without. e Representative flow cytometry plots show the differential effects of Notch inhibition with and without TNFα. d, e are mean ± standard error from n = 7 independent UCB donors and *p < 0.05.