Abstract
Objectives
To evaluate the relationship between biopsy-assessed hepatic steatosis, magnetic resonance imaging (MRI)–assessed proton density fat fraction (PDFF), and magnetic resonance elastography (MRE)–assessed liver stiffness measurement (LSM), in patients with or at risk for nonalcoholic fatty liver disease (NAFLD).
Methods
A retrospective study was performed, encompassing 256 patients who had a liver biopsy and MRI/MRE examination performed within 1 year. Clinical and laboratory data were retrieved from the electronic medical record. Hepatic steatosis and fibrosis were assessed by histopathological grading/staging. First, we analyzed the diagnostic performance of PDFF for distinguishing hepatic steatosis with the receiver operating characteristic analyses. Second, variables influencing LSM were screened with univariant analyses, then identified with multivariable linear regression. Finally, the potential relationship between PDFF and LSM was assessed with linear regression after adjustment for other influencing factors, in patients with diagnosed steatosis (PDFF ≥ 5%).
Results
The diagnostic accuracy of PDFF in distinguishing steatosis grades (S0–3) was above 0.82. No significant difference in LSM was found between patients with S1, S2, and S3 steatosis and between all steatosis grades after patients were grouped according to fibrosis stage. No statistically significant relationship was found between the LSM and PDFF (estimate = − 0.02, p = 0.065) after adjustment for fibrosis stage and age in patients with diagnosed steatosis (PDFF ≥ 5%).
Conclusions
In patients with NAFLD, the severity of hepatic steatosis has no significant influence on the liver stiffness measurement with magnetic resonance elastography.
Keywords: Magnetic resonance elastography, Liver steatosis, Liver fibrosis, Nonalcoholic fatty liver disease
Introduction
Nonalcoholic fatty liver disease (NAFLD) is an emerging epidemic, with an estimated worldwide prevalence ranging from 25 to 45% [1], increasing in parallel with obesity, diabetes, and metabolic syndrome [2]. Up to 25% of individuals with NAFLD progress to cirrhosis [3], and 7% of them to end-stage liver disease [4], demonstrating an increasing medical burden. As the histologic hallmark and a pathogenic factor in NAFLD, hepatic steatosis is characterized by an excessive intracellular accumulation of fat within hepatocytes. Other accompanying pathological changes include inflammation, hepatocyte ballooning, and fibrosis [5]. Among those pathologic features, fibrosis is the most important prognostic factor in predicting complications [2, 6]. Therefore, it is crucial to quantify liver fibrosis accurately.
Liver stiffness measurement (LSM) obtained from magnetic resonance elastography (MRE) is currently regarded as the most accurate biomarker for noninvasive liver fibrosis evaluation [7]. The diagnostic performance of MRE-based LSM for fibrosis staging has been shown to outperform other noninvasive biomarkers, including the fibrosis-4 index, NAFLD fibrosis score, the aspartate aminotransferase-to-platelet ratio index, and ultrasound elastography–based LSM [7–11].
In clinical practice, MRE assessment of liver fibrosis should take into account clinical information because other pathophysiologic conditions such as inflammation, venous congestion, portal hypertension, and cholestasis may affect liver stiffness [12]. The presence of hepatic steatosis has been reported to affect liver stiffness as measured with ultrasound-based elastography [13–15]. In contrast, most MRE-based studies have concluded that hepatic steatosis has no significant effect on MRE-assessed liver stiffness [16–18]. There are fundamental technical differences between ultrasound-based and MR-based elastography that may account for these differences. In addition, most ultrasound-based studies have used a histopathology-based assessment of steatosis, which is a subjective and semi-quantitative “gold” standard with potential errors [19, 20]. MRI-derived proton density fat fraction (PDFF) is a well-established imaging biomarker for quantifying liver steatosis on a continuous scale [21]. In clinical practice, an MRI-PDFF of 5% is often used as the threshold for diagnosing clinically significant hepatic steatosis [22].
This study aims to provide further evidence on the relationship between pathology-assessed hepatic steatosis, MRI-assessed PDFF, and MRE-assessed liver stiffness in a large patient cohort with biopsy-proven or high risk of NAFLD.
Materials and methods
Study subjects
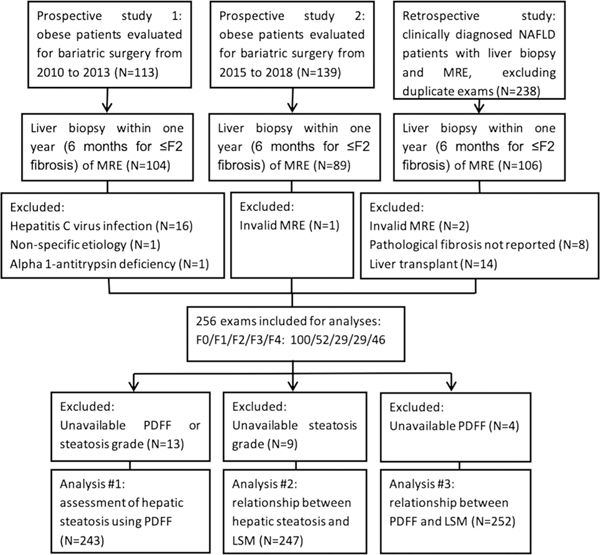
This retrospective study was approved by our Institutional Review Board, and written informed consent was waived. Patients with MRE and liver biopsy within 1 year (within 6 months for ≤ F2 fibrosis) were initially identified. We excluded patients who had liver diseases other than NAFLD, liver transplantation before MRE and biopsy, invalid MRE (failed or of poor image quality), or unavailable histologic fibrosis staging. Consequently, 256 patients were included. All patients have been previously reported in two prospective studies [23, 24] (the prospective group) or in one retrospective study [25] (the retrospective group). In the retrospective group, clinical diagnosis of NAFLD was based on a 2-step code-based algorithm, followed by individual medical record review [25]. NAFLD was defined based on the evidence of hepatic steatosis, either by imaging or by histology in the setting of risk factors such as diabetes mellitus (DM), high body mass index (BMI), or hyperlipidemia and in the absence of heavy alcohol use. The prior studies evaluated the diagnostic performance of MRE in the detection of hepatic fibrosis or nonalcoholic steatohepatitis in obese patients, and the performance of liver stiffness in predicting future cirrhosis, decompensation, and death in NAFLD. The current study focuses on the impact of hepatic steatosis/liver fat fraction on liver stiffness measurement. Detailed patient enrollment is outlined in the flowchart in Fig. 1.
Fig. 1.
Flowchart of the retrospective patient enrollment
MR imaging
The imaging protocol consisted of two-dimensional (2D) MRE to measure LSM, and 2-point or 6-point Dixon MRI to estimate PDFF. All examinations were conducted on 1.5-T MR imagers (GE Healthcare) at Mayo Clinic.
2D MRE
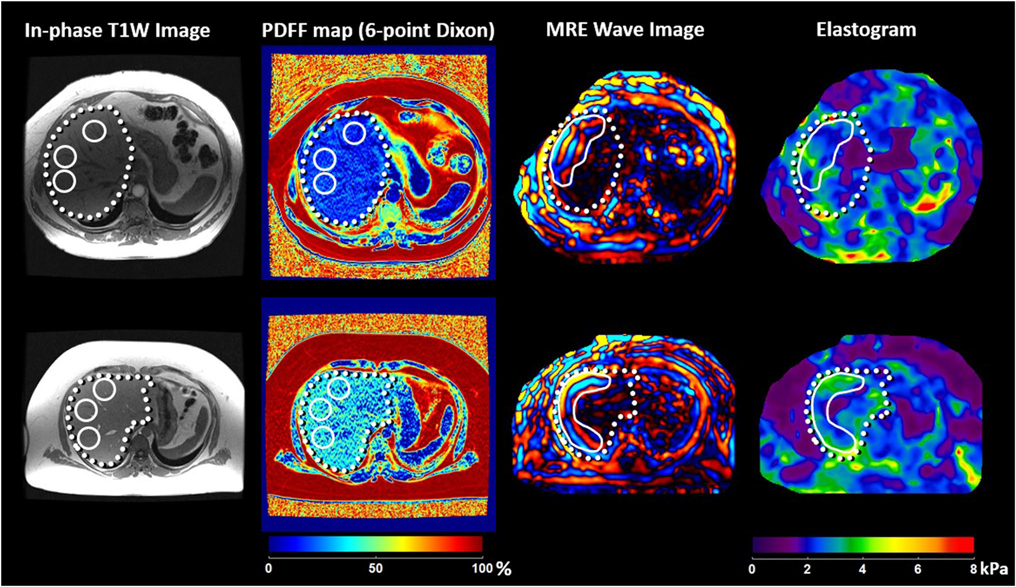
After fasting for at least 4 h, patients were imaged in the supine position with a passive pneumatic driver secured against the anterior body wall over the right hepatic lobe. Continuous acoustic waves at 60 Hz were generated from an active driver system (Resoundant, Inc.) outside the imager room and conducted into the passive driver via a flexible plastic tube. 2D MRE images were acquired in four sections with a gradient-echo MRE sequence with imaging parameters set as previously described [23]. Quantitative images depicting liver stiffness were automatically generated using the multi-model direct inversion algorithm used in all regulatory-approved implementations of MRE [26]. In all patients, analysis of the elastograms was performed manually (> 5 years’ experience) and with an automated [27] technique to yield a mean liver stiffness value. For each patient, four regions of interest were manually or automatically draw on four successive slices of the right hepatic lobe, avoiding the inclusion of artifact and major vessels (Fig. 2). Expert panel review was performed (> 10 years’ of experience) in any cases showing more than 20% difference between the two analysis methods. Final analyses were performed on the automated assessment adjusted with an expert review (if performed).
Fig. 2.
Liver stiffness and PDFF assessment in two example cases with F1 fibrosis. Mean liver stiffness was similar between two patients (2.69 kPa in the patient shown in the upper row, and 2.71 kPa in the patient shown in the bottom row), while PDFF showed a huge difference (both measured with 6-point Dixon imaging, 8.8% in the patient shown in the upper row, and 20.1% in the patient shown in the bottom row)
Dixon MRI
Final PDFF was measured from a 6-point Dixon imaging sequence (IDEAL-IQ, GE Healthcare) if available (N = 92) and from a 2-point Dixon imaging sequence otherwise (N = 160). Among 92 patients with 6-point Dixon images, 87 of them also had 2-point Dixon imaging data, and PDFF results from two methods were compared. All PDFF images were acquired during the same MRI examination as the MRE. The detailed imaging protocol for Dixon imaging with two or six echoes has been previously reported [23, 24]. For each subject, the mean value of three non-overlapped ROIs in the right lobe of the liver was recorded as the final PDFF.
Histological assessment
In the prospective group, specimen slides were reviewed by an experienced histopathologist, using the NASH CRN model [28]. In the retrospective group, pathological results using the Brunt system were retrieved from the pathology report in the electronic medical record. The histopathological fibrosis staging criteria for the NASH CRN and Brunt systems are similar [29], and therefore, data were combined for analyses. In brief, stage F0 represents no fibrosis, F1 represents perisinusoidal or portal only fibrosis, F2 represents perisinusoidal and periportal fibrosis, F3 represents bridging fibrosis, and F4 represents cirrhosis. Liver steatosis was graded according to the proportion of hepatocytes that contain microvesicles of fat as follows: grade 0 (S0) for less than 5%, grade 1 (S1) for 5–33%, grade 2 (S2) for 34–66%, and grade 3 (S3) for more than 66%. Other histopathology features that were compiled included lobular inflammation, hepatocyte ballooning, and pathologic diagnosis of NASH.
Statistical analysis
Categorical variables were summarized as numbers and percentages, and continuous variables as the median and interquartile range. Clinical and laboratory characteristics were compared between the prospective group and the retrospective group using the Kruskal–Wallis test or the chi-square test, when appropriate. The median values of PDFF and LSM at each steatosis grade were compared using the Wilcoxon multiple comparisons. Correlations between imaging measurements and physiopathological features were evaluated by Spearman’s correlation coefficient (ρ). The diagnostic performance of PDFF for hepatic steatosis was estimated using receiver operating characteristic analysis, and the optimal cut-off values were determined by maximizing Youden’s index. Variables influencing LSM were screened with univariant analyses, then identified with multivariable linear regression with backward selection based on Bayesian criteria. Locally weighted scatterplot smoothing was applied to visually depict the relationship between PDFF and LSM for all patients and each fibrosis stage, respectively. The relationship between PDFF and LSM was adjusted with identified significant influencing factors. It was confirmed with a generalized linear regression in patients with diagnosed steatosis (i.e., PDFF ≥ 5%) to exclude two extremes: (1) obese patients at a high risk of developing NAFLD but not developing steatosis yet; (2) end-stage fibrosis with “burned-out” steatosis. Subgroup analyses were performed in patients with 6-point Dixon imaging data, which are believed to have a more accurate PDFF measurement, and in patients with a LSM < 5 kPa, which are considered less likely to have cirrhosis [11, 23]. Linear correlation and Bland–Altman plots were calculated between paired PDFF measurements in patients with both the 2- and 6-point Dixon imaging data available. Linear regression with additional dichotomous variables of sex and PDFF method was also performed to compare the influence of these factors on the result. Statistical analyses were supervised by a senior statistician (T.T.) and performed by one of the researchers (J.C.), using both R statistical software (R Foundation for Statistical Computing, version 3.5.3) and JMP (JMP@ Pro 14.1.0). A significance threshold of p < 0.05 was selected.
Results
Demographic, clinic, and pathologic characteristics
Fibrosis stages were determined as F0 in 100 patients, F1 in 52 patients, F2 in 29 patients, F3 in 29 patients, and F4 in 46 patients. Hepatic steatosis was determined as S0 in 44 patients, S1 in 122 patients, S2 in 51 patients, S3 in 30 patients, and not reported in 9 patients. There were statistically significant differences in the clinical and histopathological characteristics between the prospective and the retrospective groups. Patients from the prospective group had a higher body mass index (median: 42.92 kg/m2 versus 36.00 kg/m2), and a lower fibrosis stage, steatosis grade, inflammation, and ballooning (p < 0.05 for all). The median value of PDFF did not show a significant difference between the two groups. The median time interval between imaging study and biopsy was 20 days (range 0 to 344 days) overall and 16 days (range 1 to 99 days) for the prospective group. Detailed demographic, clinical, and pathologic characteristics for the two groups and comparison results are shown in Table 1.
Table 1.
Demographic, clinic, and pathologic information of included patients in the prospective and the retrospective group
| Prospective group (N = 174) | Retrospective group (N = 82) | p | |
|---|---|---|---|
| Age | 47 (39, 56) | 60 (55, 67) | < 0.001 |
| Male | 45 (25.86%) | 31 (37.80%) | 0.058 |
| Body mass index | 42.92 (39.29, 47.46) | 36.00 (29.50, 40.74) | < 0.001 |
| Albumin | 4.2 (4.1, 4.4) | 4.0 (3.5, 4.3) | < 0.001 |
| N missing | 61 | 5 | |
| Aspartate aminotransferase | 28 (22, 39.5) | 53.5 (34, 77) | < 0.001 |
| N missing | 33 | 0 | |
| Alanine aminotransferase | 32.5 (22, 54) | 49 (33, 82.75) | < 0.001 |
| N missing | 48 | 0 | |
| Alkaline phosphatase | 82 (67, 96) | 99 (72.25, 138.75) | 0.003 |
| N missing | 115 | 2 | |
| Hemoglobin | 12.7 (11.6, 13.9) | 13.6 (12.2, 14.25) | 0.031 |
| N missing | 4 | 1 | |
| Hematocrit | 38.8 (35.88, 41.58) | 40.1 (35.9, 42.95) | 0.411 |
| N missing | 8 | 41 | |
| Leukocytes | 9 (7.48, 11.2) | 6.8 (4.8, 14.2) | < 0.001 |
| N missing | 8 | 41 | |
| Platelet | 237.5 (199.5, 283) | 174 (127.75, 242.25) | < 0.001 |
| N missing | 8 | 0 | |
| Total bilirubin | 0.5 (0.4, 0.7) | 0.8 (0.5, 1.5) | < 0.001 |
| N missing | 127 | 6 | |
| Total cholesterol | 166.5 (142.25, 197) | 159 (127, 199) | 0.467 |
| N missing | 86 | 23 | |
| Triglycerides | 157 (103, 220.75) | 143 (93.75, 180.5) | 0.231 |
| N missing | 88 | 22 | |
| Creatinine | 0.8 (0.7, 1) | 0.9 (0.7, 1.1) | 0.020 |
| N missing | 26 | 3 | |
| Fibrosis: F0/F1/F2/F3/F4 | 94/44/18/8/10 | 6/8/11/21/36 | < 0.001 |
| Steatosis: S0/S1/S2/S3 | 38/86/31/18 | 6/36/20/12 | 0.028 |
| N missing | 1 | 8 | |
| Lobular inflammation: A0/A1/A2/A3 | 66/92/14/1 | 9/43/20/1 | < 0.001 |
| N missing | 1 | 9 | |
| Ballooning: B0/B1/B2 | 105/51/17 | 6/35/18 | < 0.001 |
| N missing | 1 | 23 | |
| Nonalcoholic steatohepatitis | 76 (43.93%) | 63 (82.89%) | < 0.001 |
| N missing | 1 | 6 | |
| Proton density fat fraction (%) | 9.88 (4.96, 18.07) | 11.19 (2.62, 16.55) | 0.360 |
| N missing | 1 | 3 |
Categorical variables were summarized as numbers and percentages, and continuous variables as the median and interquartile range. N missing = number of cases with corresponding missing information. Data were compared between the prospective group and the retrospective group using the Kruskal–Wallis test or the chi-square test when appropriate
Assessment of hepatic steatosis using PDFF
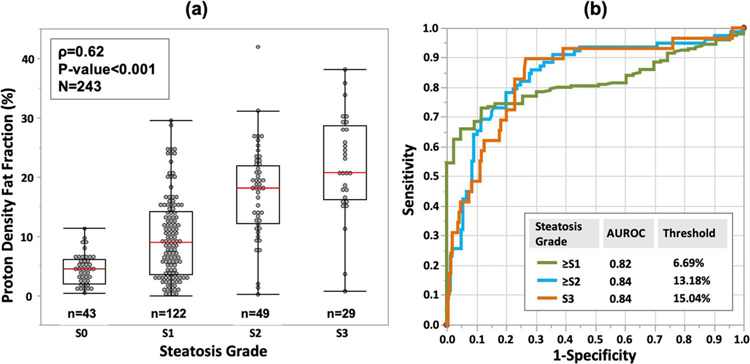
PDFF correlated significantly with steatosis grades (Fig. 3a, ρ = 0.62, p < 0.001). Median PDFF (interquartile range) was 4.41% (2–6.03) in S0, 8.95% (3.57–14.11) in S1, 18.03% (12.14–21.97) in S2, and 20.8% (16.13–28.69) in S3. The areas under the receiver operating characteristic curve (AUROC) with corresponding 95% confidence intervals for predicting ≥ S1, ≥ S2, and S3 steatosis were 0.82 (0.77, 0.87), 0.84 (0.79, 0.90), and 0.84 (0.75, 0.92), respectively, with corresponding cutoff values of 6.69%, 13.18%, and 15.04% in PDFF (Fig. 3b).
Fig. 3.
a Proton density fat fraction measurement increases with steatosis grade. Red lines indicate median values. b Receiver operating characteristic analyses of predicting hepatic steatosis grade using proton density fat fraction
Excellent correlation (r = 0.97, p < 0.001, Supplementary Fig. 1) and statistically significant bias (− 3.8%, p < 0.001, Supplementary Fig. 2) were observed between PDFF measurements (range: 0–42%) from paired 2- and 6-point Dixon images in 87 patients.
Relationship between histopathologic findings and LSM
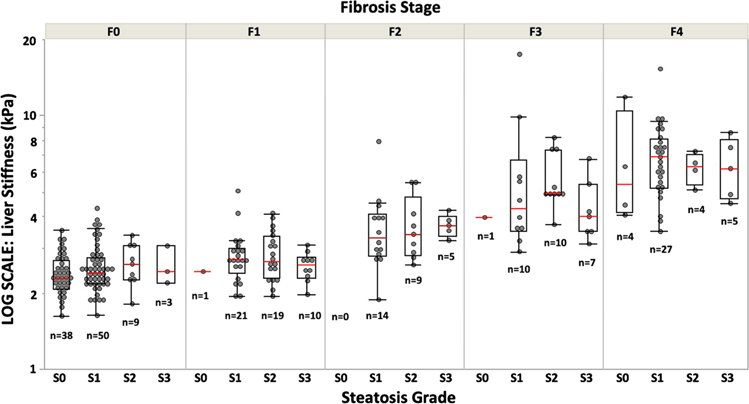
The following physiological and histopathologic features correlated significantly with LSM: age (ρ = 0.42, p < 0.001), body mass index (ρ = − 0.41, p < 0.001), fibrosis stage (ρ = 0.79, p < 0.001), biopsy-based steatosis grade (ρ = 0.26, p < 0.001), lobular inflammation (ρ = 0.44, p < 0.001), and hepatocyte ballooning (ρ = 0.57, p < 0.001). Multivariable linear regression with backward selection based on Bayesian information showed that only age (estimate = 0.02, p = 0.018), fibrosis stage (estimate = 1.05, p < 0.001), and biopsy-based steatosis grade (estimate = − 0.33, p = 0.005) were significant covariates influencing LSM. Median LSM (interquartile range) was 2.34 (2.10–2.90) kPa in S0, 2.93 (2.39–4.67) kPa in S1, 3.19 (2.58–4.90) kPa in S2, and 3.36 (2.61–4.31) kPa in S3. Patients without histopathologic steatosis (S0) showed significantly lower LSM than patients with steatosis (S1, S2, and S3). No significant difference in LSM was found between patients with S1, S2, and S3 steatosis. After grouping subjects according to fibrosis stage, there were no significant differences in LSM between steatosis grades within each fibrosis stage (Fig. 4).
Fig. 4.
Distribution of MRE-based liver stiffness measurement (LSM) according to histologic steatosis grade and fibrosis stage. Red lines indicate median values. There was no significant difference in LSM between steatosis grades within each fibrosis stage
Relationship between PDFF and LSM
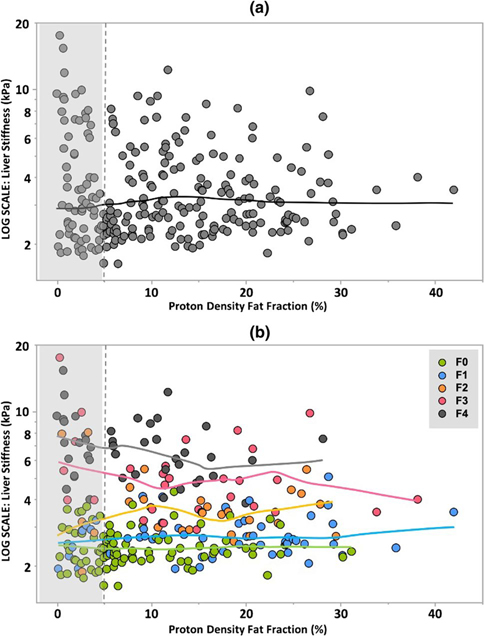
The locally smoothed plot of LSM versus PDFF revealed little evidence of substantial relationships between LSM and PDFF (Fig. 5a). When the data are grouped by fibrosis stage, the fitted curves for each stage of fibrosis also showed little visual evidence of a systematic relationship between quantitative measurements of liver fat and liver stiffness (Fig. 5b). The results of generalized linear regression with fibrosis stage, age, and PDFF as independent covariates and LSM as the dependent variable in patients with diagnosed steatosis (PDFF ≥ 5%) confirmed no statistically significant relationship between LSM and PDFF (estimate = − 0.02, p = 0.065). Subgroup analyses in patients with 6-point Dixon imaging, or with LSM < 5 kPa, also revealed no significant relationship between LSM and PDFF (estimate = − 0.004 and − 0.003, respectively, p > 0.05 for both). The results of the linear regressions are summarized in Table 2. Also, the specific PDFF method used (2-point Dixon versus 6-point Dixon) showed no significant influence on the conclusion (Supplementary Table 1). Example images of the liver with the same fibrosis stage and liver stiffness but different PDFF values are demonstrated in Fig. 2.
Fig. 5.
a Distribution of MRE-based liver stiffness measurements and MRI-based proton density fat fraction in the entire cohort. Unshaded region denotes the patient with hepatic steatosis. The fitted curve demonstrates no substantial relationship between liver stiffness and PDFF for the entire cohort. b Fitted curves for F0–F4 fibrosis demonstrate no evidence of a substantial systematic or monotonic relationship between MRE-based liver stiffness measurements and PDFF
Table 2.
Results of the linear regressions with liver stiffness measurement as the dependent variable in patients with diagnosed steatosis (PDFF ≥ 5%), and in different subgroups
| Covariates | Estimate | Chi-square Likelihood | LogWorth | p value # |
|---|---|---|---|---|
|
| ||||
| Patients with steatosis (N=179) | ||||
| Fibrosis stage | 0.92 | 144.96 |

|
<0.001 |
| Age | 0.02 | 8.00 | 0.005 | |
| PDFF (%) | −0.02 | 3.39 | 0.065 | |
|
Patients with steatosis and LSM<5kPa (N=148) | ||||
| Fibrosis stage | 0.46 | 95.56 |
|
<0.001 |
| Age | 0.009 | 5.85 | 0.016 | |
| PDFF (%) | −0.003 | 0.34 | 0.559 | |
|
Patients with steatosis and six-point Dixon imaging (N=76) | ||||
| Fibrosis stage | 0.61 | 67.42 |

|
<0.001 |
| Age | 0.003 | 0.40 | 0.525 | |
| PDFF (%) | −0.004 | 0.30 | 0.583 | |
PDFF, proton density fat fraction; Blue vertical line = significance level of 0.05
p value is for the test of the estimates
Discussion
This study evaluated the relationship between LSM and hepatic steatosis in a large NAFLD population. The results of this cross-sectional study indicate that in patients with NAFLD, the severity of hepatic steatosis, as assessed quantitively with MRI-PDFF methods, does not have a significant effect on MRE-assessed liver stiffness. After patients were stratified according to fibrosis stage, no significant difference was found in LSM between all steatosis grades. The results provide evidence that previous reports of an apparent relationship between LSM and biopsy-based histologic grading of hepatic stiffness described in ultrasound-based elastography studies reflect the biologic association of steatosis and fibrosis rather than a significant physical measurement relationship between hepatic fat content and LSM.
While the fitted curves for each stage of fibrosis shown in Fig. 4b show no substantial systematic relationships between LSM and PDFF, there is a modest downward trend for the F3 and F4 curves with increasing PDFF. However, the figure shows that the patients with most advanced fibrosis are clustered in the upper left quadrant of the figure. The substantially decreased or absence of steatosis in patients with advanced fibrosis is known as “burned-out” NASH [18], characterized as the extracellular matrix gradually replaces viable hepatocytes that accumulate lipid droplets as fibrosis progresses. This spectrum effect in the study cohort may account for the slight downward trend of the F3 and F4 curves in Fig. 4b.
The results of this study are consistent with most other publications that have concluded that the severity of hepatic steatosis has little effect on MRE-based LSM [17, 18, 30]. Chen et al. [18] first reported a non-significant correlation between PDFF and LSM. Other studies using MRE [17, 31] or ultrasound-based elastography [30, 31] also suggested that fibrosis was the major variable associated with LSM, and steatosis was not.
We speculate that linked histopathologic processes such as inflammation which affect stiffness may explain the apparent correlation between LSM and hepatic steatosis that has been reported in a minority of studies [13–16]. In addition, fibrosis stage was treated mainly as a dichotomous factor in some of these studies [13–15].
Our study had some limitations. First, patients with different characteristics were combined to increase the sample size. Together, our data better represents the entire disease spectrum of NAFLD. Second, PDFF was derived from either 2- or 6-point Dixon imaging depending on availability (2-point Dixon, 64%). In the supplementary material, we included a side analysis of the accuracy of the 2-point Dixon–derived PDFF and the impact of PDFF method in the LSM by considering it as an additional confounding factor.
In summary, this study confirms that after adjustment for fibrosis stage and other independent factors, the severity of hepatic steatosis has no significant effect on MRE-based liver stiffness measurement.
Supplementary Material
Key Points.
The MRI-based proton density fat fraction provides a quantitative assessment of hepatic steatosis with high accuracy.
No significant effect of hepatic steatosis on MRE-based liver stiffness measurement was found in patients with S1, S2, and S3 steatosis and between all steatosis grades after patients were grouped according to fibrosis stage.
After adjusting for fibrosis stage and age, there was no statistically significant relationship between liver stiffness and proton density fat fraction in patients with hepatic steatosis (p = 0.065).
Acknowledgements
This work has been supported by grant funding from the National Institutes of Health: R37 EB001981 (R.L.E.), R01 EB017197 (M.Y.), K23 DK115594 (A.M.A.), and the US Department of Defense: W81XWH-19-1-0583-01 (M.Y.). The author would like to thank Jennifer Kugel for providing language help for this manuscript.
Funding This study has received grant funding from the National Institutes of Health: R37 EB001981 (R.L.E.), R01 EB017197 (M.Y.), K23 DK115594 (A.M.A.), and the US Department of Defense: W81XWH-19-1-0583-01 (M.Y.).
Abbreviations
- AUROC
Areas under the receiver operating characteristic curve
- LSM
Liver stiffness measurement
- MRE
Magnetic resonance elastography
- NAFLD
Nonalcoholic fatty liver disease
- NASH
Nonalcoholic steatohepatitis
- PDFF
Proton density fat fraction
Footnotes
Conflict of interest The Mayo Clinic, Jun Chen, Richard L. Ehman, and Meng Yin have intellectual property and a financial interest related to our research in this work.
Declarations
Guarantor The scientific guarantor of this publication is Richard L. Ehman (ehman.richard@mayo.edu,Tel: 1-(507)-284-9781, 200 First Street SW, Rochester, Minnesota 55905, United States).
Statistics and biometry Dr. Terry Therneau provided statistical advice and supervised Dr. Jie Chen to perform the statistical analyses.
Informed consent Written informed consent was waived by the Institutional Review Board.
Ethical approval Institutional Review Board approval was obtained.
- retrospective
- diagnostic or prognostic study
- performed at one institution
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00330-021-08225-w.
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M (2016) Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64:73–84 [DOI] [PubMed] [Google Scholar]
- 2.Lindenmeyer CC, McCullough AJ (2018) The Natural History of Nonalcoholic Fatty Liver Disease-An Evolving View. Clin Liver Dis 22:11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Önnerhag K, Nilsson PM, Lindgren S (2014) Increased risk of cirrhosis and hepatocellular cancer during long-term follow-up of patients with biopsy-proven NAFLD. Scand J Gastroenterol 49:1111–1118 [DOI] [PubMed] [Google Scholar]
- 4.Ekstedt M, Franzén LE, Mathiesen UL et al. (2006) Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 44:865–873 [DOI] [PubMed] [Google Scholar]
- 5.Pais R, Pascale A, Fedchuck L, Charlotte F, Poynard T, Ratziu V (2011) Progression from isolated steatosis to steatohepatitis and fibrosis in nonalcoholic fatty liver disease. Clin Res Hepatol Gastroenterol 35:23–28 [DOI] [PubMed] [Google Scholar]
- 6.Angulo P, Kleiner DE, Dam-Larsen S et al. (2015) Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 149:389–397.e310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G (2017) Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: a meta-analysis. Hepatology 66:1486–1501 [DOI] [PubMed] [Google Scholar]
- 8.Staufer K, Halilbasic E, Spindelboeck W et al. (2019) Evaluation and comparison of six noninvasive tests for prediction of significant or advanced fibrosis in nonalcoholic fatty liver disease. United European Gastroenterol J 7:1113–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu XY, Wang WS, Zhang QM et al. (2019) Performance of common imaging techniques vs serum biomarkers in assessing fibrosis in patients with chronic hepatitis B: a systematic review and meta-analysis. World J Clin Cases 7:2022–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imajo K, Kessoku T, Honda Y et al. (2016) Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology 150:626–637.e627 [DOI] [PubMed] [Google Scholar]
- 11.Park CC, Nguyen P, Hernandez C et al. (2017) Magnetic resonance elastography vs transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy-proven nonalcoholic fatty liver disease. Gastroenterology 152:598–607.e592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang A, Cloutier G, Szeverenyi NM, Sirlin CB (2015) Ultrasound elastography and MR elastography for assessing liver fibrosis: Part 2, Diagnostic performance, confounders, and future directions. AJR Am J Roentgenol 205:33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petta S, Maida M, Macaluso FS et al. (2015) The severity of steatosis influences liver stiffness measurement in patients with non-alcoholic fatty liver disease. Hepatology 62:1101–1110 [DOI] [PubMed] [Google Scholar]
- 14.Shen F, Mi YQ, Xu L et al. (2019) Moderate to severe hepatic steatosis leads to overestimation of liver stiffness measurement in chronic hepatitis B patients without significant fibrosis. Aliment Pharmacol Ther 50:93–102 [DOI] [PubMed] [Google Scholar]
- 15.Karlas T, Petroff D, Sasso M et al. (2018) Impact of controlled attenuation parameter on detecting fibrosis using liver stiffness measurement. Aliment Pharmacol Ther 47:989–1000 [DOI] [PubMed] [Google Scholar]
- 16.Joshi M, Dillman JR, Singh K et al. (2018) Quantitative MRI of fatty liver disease in a large pediatric cohort: correlation between liver fat fraction, stiffness, volume, and patient-specific factors. Abdom Radiol (NY) 43:1168–1179 [DOI] [PubMed] [Google Scholar]
- 17.Leitão HS, Doblas S, Garteiser P et al. (2017) Hepatic Fibrosis, Inflammation, and Steatosis: Influence on the MR Viscoelastic and Diffusion Parameters in Patients with Chronic Liver Disease. Radiology 283:98–107 [DOI] [PubMed] [Google Scholar]
- 18.Chen J, Talwalkar JA, Yin M, Glaser KJ, Sanderson SO, Ehman RL (2011) Early detection of nonalcoholic steatohepatitis in patients with nonalcoholic fatty liver disease by using MR elastography. Radiology 259:749–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ratziu V, Charlotte F, Heurtier A et al. (2005) Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 128:1898–1906 [DOI] [PubMed] [Google Scholar]
- 20.Longerich T, Schirmacher P (2020) Determining the reliability of liver biopsies in NASH clinical studies. Nat Rev Gastroenterol Hepatol 17:653–654 [DOI] [PubMed] [Google Scholar]
- 21.Reeder SB, Sirlin CB (2010) Quantification of liver fat with magnetic resonance imaging. Magn Reson Imaging Clin N Am 18:337–357 (ix) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu J, Liu S, Du S et al. (2019) Diagnostic value of MRI-PDFF for hepatic steatosis in patients with non-alcoholic fatty liver disease: a meta-analysis. Eur Radiol 29:3564–3573 [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Yin M, Talwalkar JA et al. (2017) Diagnostic performance of MR elastography and vibration-controlled transient elastography in the detection of hepatic fibrosis in patients with severe to morbid obesity. Radiology 283:418–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen AM, Shah VH, Therneau TM et al. (2020) The role of three-dimensional magnetic resonance elastography in the diagnosis of nonalcoholic steatohepatitis in obese patients undergoing bariatric surgery. Hepatology 71:510–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gidener T, Ahmed OT, Larson JJ et al. (2020) Liver stiffness by magnetic resonance elastography predicts future cirrhosis, decompensation and death in NAFLD. Clin Gastroenterol Hepatol. 10.1016/j.cgh.2020.09.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silva AM, Grimm RC, Glaser KJ et al. (2015) Magnetic resonance elastography: evaluation of new inversion algorithm and quantitative analysis method. Abdom Imaging 40:810–817 [DOI] [PubMed] [Google Scholar]
- 27.Dzyubak B, Venkatesh SK, Manduca A, Glaser KJ, Ehman RL (2016) Automated liver elasticity calculation for MR elastography. J Magn Reson Imaging 43:1055–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kleiner DE, Brunt EM, Van Natta M et al. (2005) Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 41(6):1313–21. 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 29.Brunt EM (2016) Nonalcoholic fatty liver disease: pros and cons of histologic systems of evaluation. Int J Mol Sci 13;17(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eddowes PJ, Sasso M, Allison M et al. (2019) Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology 156:1717–1730 [DOI] [PubMed] [Google Scholar]
- 31.Hudert CA, Tzschätzsch H, Rudolph B et al. (2021) How histopathologic changes in pediatric nonalcoholic fatty liver disease influence in vivo liver stiffness. Acta Biomater 123:178–186 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.