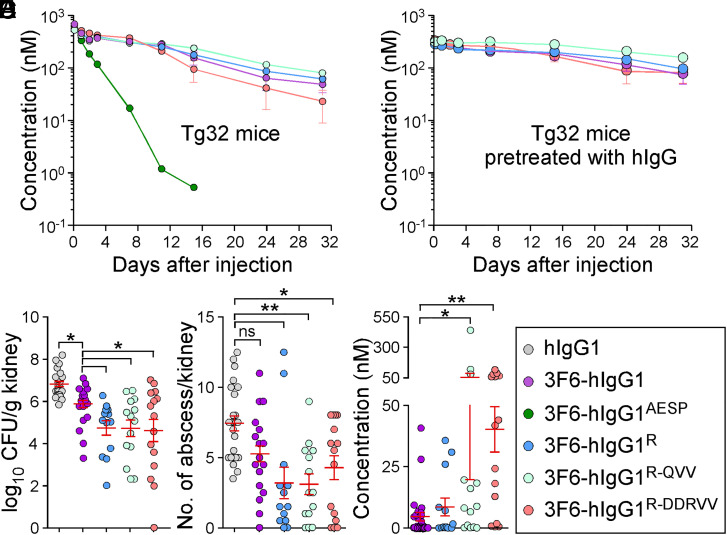
Fig. 6.
Antibody efficacy in the humanized FcRn (Tg32) mice. (A and B) Serum concentrations over time of 3F6-hIgG1R and variants R-QVV and R-DDRVV following injection (5 mg/kg) in Tg32 mice without (A) or with (B) pretreatment with pooled hIgG at 100 mg/kg (n = 5 mice per group). (C and D) Enumeration of bacterial loads (C) and kidney surface abscesses (D) as described in Fig. 3 in Tg32 animals pretreated with antibody hIgG1, 3F6-hIgG1, 3F6-hIgG1R, 3F6-hIgG1R-QVV, or 3F6-hIgG1R-DDRVV challenged for 15 d with S. aureus MW2 (n = 13 to 17 mice). (E) Serum concentration of test antibodies that remained after 15 d infection with S. aureus MW2. Sera were from animals shown in C. (F) Color code for test antibodies used in this figure. Data are presented as mean ± SEM and analyzed with one-way ANOVA and Tukey’s multiple-comparison test in C–E (**P < 0.01; *P < 0.05).