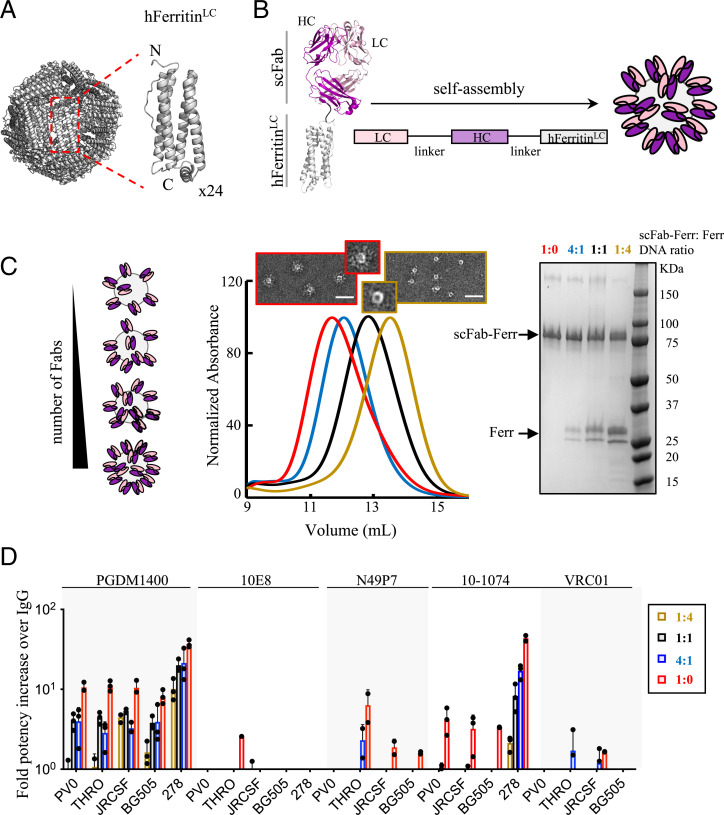
Fig. 1.
HIV-1 bNAb multimerization increases neutralization potency. Schematic of the self-assembly of (A) apoferritin (24 subunits) and (B) scFab-apoferritin fusions. Fab light chain (LC) and heavy chain (HC) are shown in light and dark pink, respectively, and are connected to the N terminus of the light chain of human apoferritin (gray) through a glycine-glycine-serine–like flexible linker (black). (C) Schematic representation of different Fab densities displayed on human apoferritin. Cotransfection of scFab-human apoferritin–encoding plasmids together with unconjugated apoferritin at ratios of 1:4 (dark yellow), 1:1 (black), 4:1 (blue), and 1:0 (red) resulted in molecules with different scFab valency, as confirmed by elution volumes in size exclusion chromatography (SEC) and less unconjugated apoferritin in sodium dodecyl sulfate polyacrylamide gel electrophoresis. Negative-stain electron micrographs of the samples with the lowest and highest scFab valency are shown. (Scale bar, 50 nm.) (D) Avidity effect on neutralization of five bNAbs against a five-PsV panel (PVO.04, JRCSF, BG505 T332N, THRO4156.18, and t278-50). Fold potency increase was calculated as the parental IgG IC50 (micrograms per milliliter) divided by the Fab-apoferritin fusion IC50 (micrograms per milliliter). Fold potency increase analyses were omitted in the following cases: N49P7-t278-50, VRC01-T278-50, and 10-1074-THRO4156.18 due to neutralization resistance. Bars (± SD) represent the mean value from n = 3 biologically independent samples.