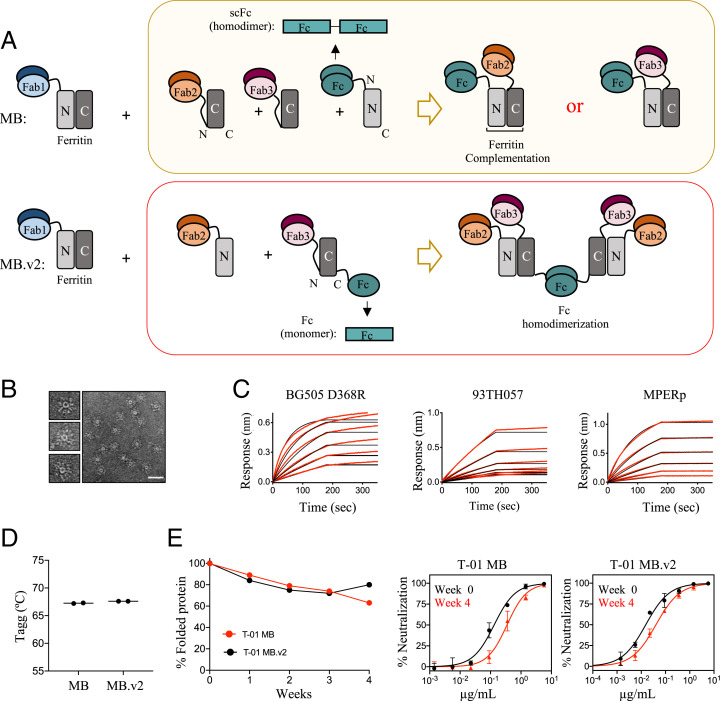
Fig. 3.
Engineering and biophysical characterization of Multabody v2. (A) The second-generation Multabody design displays two distinct features in comparison to the original Multabody design: 1) The Fc (green) is fused to the C terminus of the second half of apoferritin in the split ferritin design, and 2) the scFc domain (green), fused to the C terminus of an apoferritin half protomer, is reverted to a monomeric Fc chain. Dimerization of each Fc in MB.v2 drives assembly of four Fabs (two Fab2 and two Fab3, Bottom) while only one Fab is assembled per Fc into the previous MB version (Top). (B) Negative-stain electron micrographs of T-01 MB.v2. (Scale bar, 50 nm.) (C) Concentration-response curves for binding of T-01 MB.v2 to multiple epitopes. Red lines represent raw data; black lines represent global fits. (D) Comparison of Tagg and (E) long-term stability under temperature stress conditions (10 mg/mL; 40 °C) of the two different Multabody versions. PsV neutralization (mean values ± SD for two technical replicates) comparison at week 0 vs. week 4 is shown.