Fig. 7. Calcineurin inhibition does not affect OmpX vaccine efficacy and lung Th17 cells.
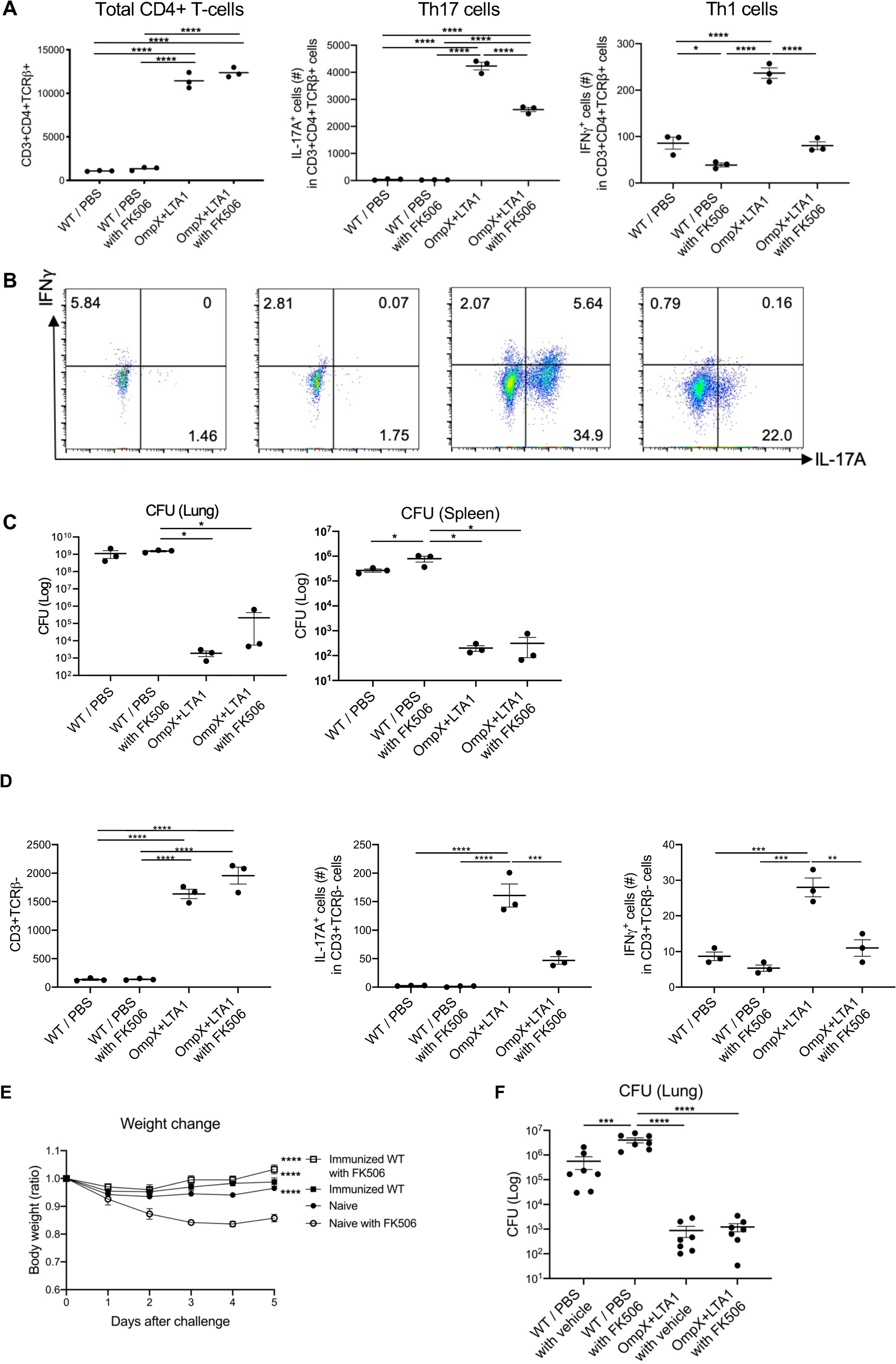
(A) Total lung CD3+CD4+ T cells, IL-17A+ cells (Th17 cells) and IFNγ+ cells (Th1 cells) in naïve C57BL/6 mice and OmpX+LTA1 vaccinated mice after treatment with FK506 or vehicle (intraperitoneal dosing from 24 h prior to challenge, followed two doses 24 h apart). (n = 3). (B) Representative dot plots of IL-17A+ and IFNγ+ lung CD3+CD4+TCRβ+ T cells in each group are shown. (C) Lung and spleen CFU in naïve and OmpX+LTA1 vaccinated C57BL/6 mice, with or without FK506 treatment, were estimated at 24 h post infection (n = 3–4). Data are representative from two independent experiments. (D) Total lung γδ cells, IFNγ+ and IL-17A+ cells in naïve and OmpX+LTA1 vaccinated after treatment with FK506 or vehicle (intraperitoneal dosing from 24 h prior to challenge, followed two doses 24 h apart). (n = 3). (E, F) Vaccine efficacy in a KPC-producing K.pneumoniae-challenge model using 107 CFU of a mucoid I1 (ST258 strain), in the presence or absence of 10 mg/kg FK506. (E) Weight change over time (D, n = 6 per group) and (F) lung CFU (E, n = 7 per group) are shown. Data are pooled from two independent experiments and presented as mean ± SEM. Significant differences were calculated with one-way ANOVA followed by Tukey’s multiple comparisons test. *, P < 0.05; ****, P < 0.0001
