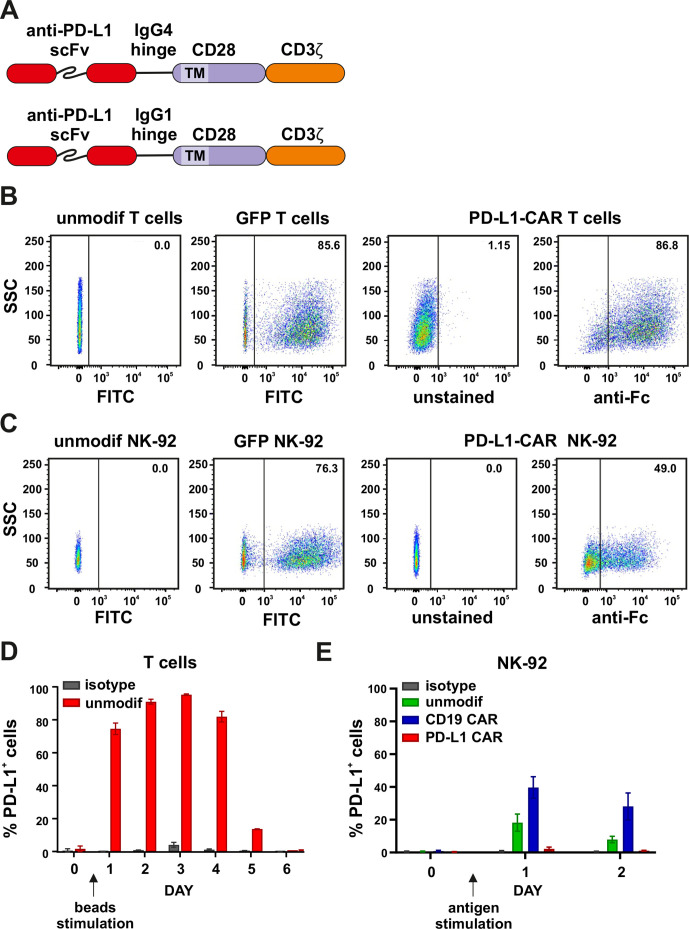
Figure 2.
Generation and expression of PD-L1–CAR in immune effector cells. (A) The scheme depicting the modular structure of PD-L1–CARs used in the study (in detail described in the Materials and methods section). (B) Flow cytometry analysis of GFP (left panels) or PD-L1–CAR (right panels) expression in T cells after lentiviral transduction. GFP expression was detected in FITC channel and PD-L1–CAR expression was detected using anti-human IgG, Fcγ fragment specific antibody (cat. no. 109-606-098, Jackson ImmunoResearch). Numbers on the density plots indicate the percentage of PD-L1–CAR-positive cells. The experiments were repeated at least three times. (C) Flow cytometry analysis of GFP (left panels) or PD-L1–CAR (right panels) expression in NK-92 cells after lentiviral transduction was performed as described in (B). (D) PD-L1 expression on primary T cells. Bar graphs represent PD-L1 expression on unmodified effector cells T cells. T cells were cultivated in the presence of 100 U/mL of IL-2 alone (day 0) or together with human T-activator CD3/CD28 beads (days 1–6). Day 1 represents the first day after the stimulation of T cells with human T-activator CD3/CD28 beads. PD-L1 staining was performed on consecutive days using an anti-PD-L1 antibody (clone 29E.2A3, dilution 1:100). The experiment was repeated in duplicates two times. (E) PD-L1 expression on NK-92 cell line. Flow cytometry analysis of PD-L1 expression in NK-92, CD19–CAR NK-92, and PD-L1–CAR NK-92 cells following the stimulation with target Raji PD-L1 cells. The effector cells were coincubated with targets in a 1:1 E:T ratio. The PD-L1 expression on NK-92, CD19–CAR and PD-L1–CAR NK-92 cells was assessed 24 and 48 hours after the stimulation using an anti-PD-L1 antibody (clone MIH1, cat. no. 14-5983-82, eBioscience, diluted 1:100). Bar graphs represent the percentage of PD-L1-positive cells. The experiment was repeated three times. CAR, chimeric antigen receptor, PD-L1, programmed death-ligand 1, GFP, green fluorescent protein, FITC, fluorescein isothiocynate, scFV, single-chain variable fragment