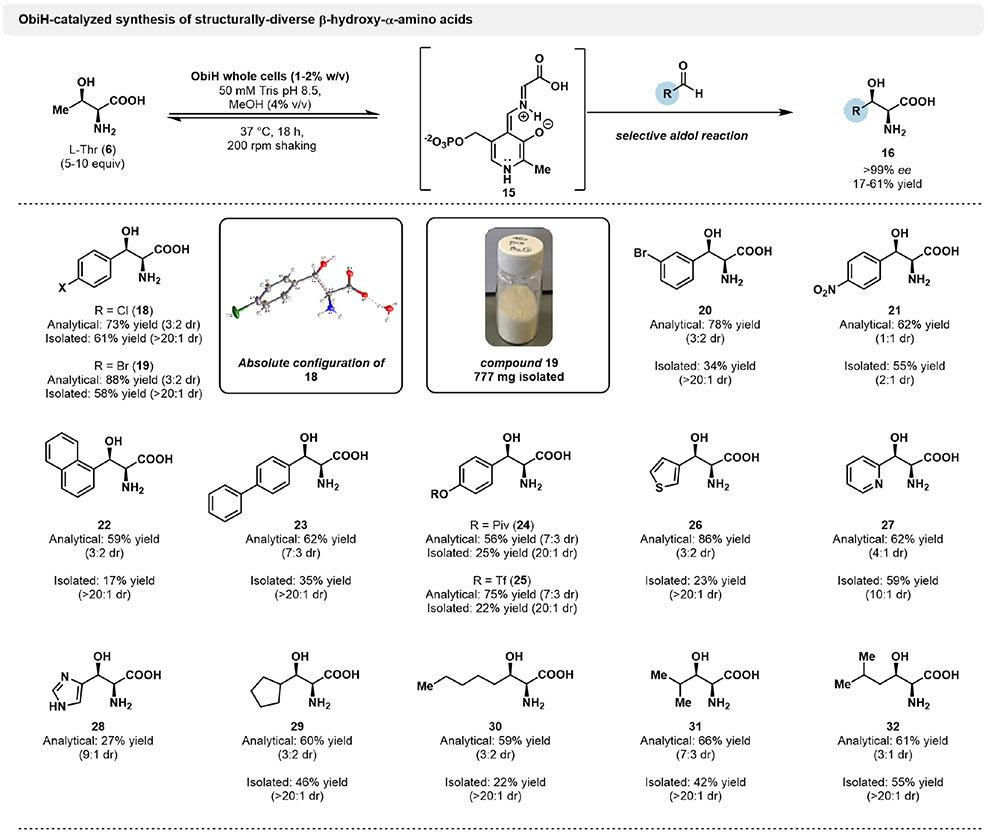
Figure 2.
Analytical and preparative-scale synthesis of β-hydroxy-α-amino acids by ObiH. Reactions were performed using 20 mM aldehyde, 100 mM l-Thr, 50 mM Tris pH 8.5 and 1–2% ObiH wet whole cells, with 4% (v/v) MeOH as co-solvent. Preparative scale reactions were incubated at 37 °C for 18 h before quenching with 1 volume equivalent of MeCN, followed by freeze-thaw and centrifugation to remove cell debris. Purification was achieved using a Biotage purification system via reverse-phase chromatography. Yields are reported as isolated product mass after lyophilization. 1H NMR hydration analysis was used to correct yield values for excess water. Analytical scale product yields determined by UPLC-PDA-MS following derivatization with Marfey’s reagent.