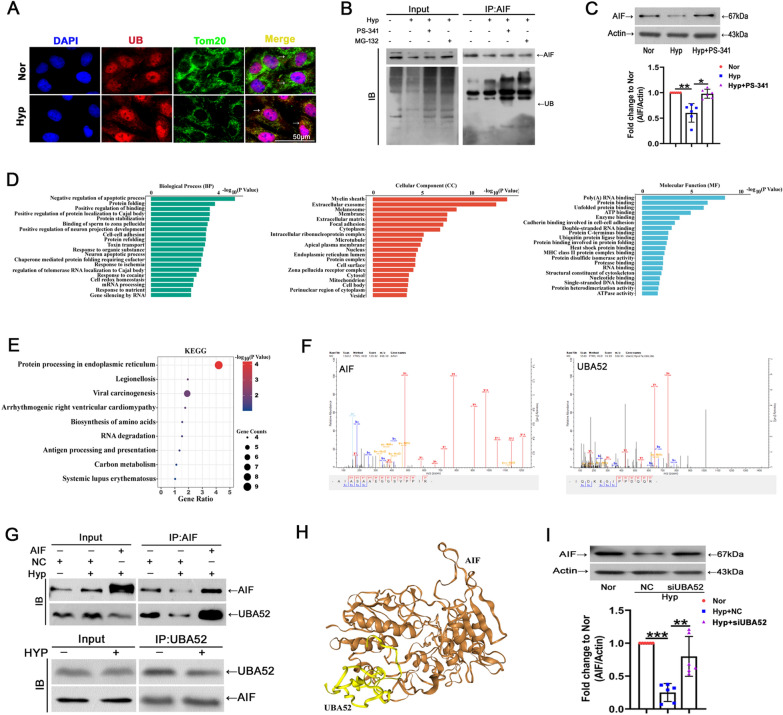
Fig. 6.
UBA52 participates in AIF ubiquitination, leading to its degradation by the proteasome system. A The colocalization of ubiquitin (UB) and Tom20 was determined using immunofluorescence (n = 3). Scale bars: 50 μm. B PASMCs were exposed to normoxia or hypoxia for 24 h, and co-IP assay was performed using anti-AIF, followed by probing with anti-UB (n = 3). C Cells were treated with or without PS-341 for 24 h, and the expression levels of AIF and β-actin were examined (n = 6). D, E GO and KEGG analysis of proteins interacting with AIF. F Mass spectrometry of specific segments of AIF and UBA52. G After PASMCs were exposed to normoxia or hypoxia, whole cell lysates were extracted for co-IP assay with anti-AIF or anti-UBA52, followed by probing with anti-UBA52 or anti-AIF (n = 3). H Representative predicted binding sites and structures of UBA52 and AIF. I PASMCs were transfected with si-UBA52 and then exposed to hypoxia, and the protein expression of AIF was estimated with β-actin serving as the standard (n = 6). All data are presented as the means ± standard deviation. *p < 0.05; **p < 0.01; ***p < 0.001; Nor normoxia, Hyp hypoxia, NC negative control, si small interfering RNA