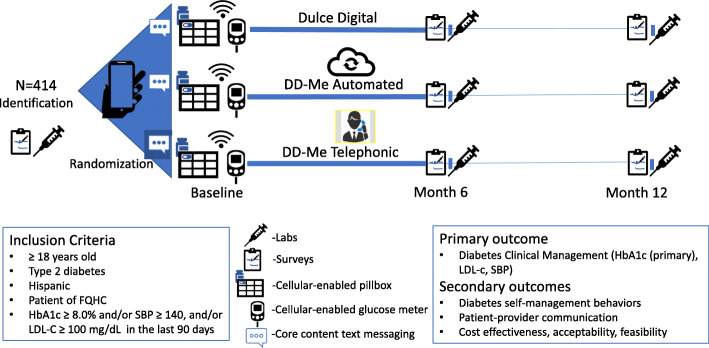
Fig. 1.
DD-Me trial design. After identification and randomization, all participants are provided a cellular-enabled pill box, glucose monitor and (if needed) mobile phone. All received Core Content text messages and were asked to answer ecological momentary assessments (EMA) over 6 months. Pill box, glucose levels, and EMA item responses were used in the Dulce Digital-Me-Automated and Dulce Digital-Me-Telephonic groups to formulate adaptive messaging during the 6-month intervention period. Follow-up labs and surveys were conducted at months 6 and 12. DD-Me-Dulce Digital-Me, FQHC-Federally Qualified Health Center, HbA1c-glycosylated hemoglobin, LDL-c low-density lipoprotein cholesterol, SBP-systolic blood pressure