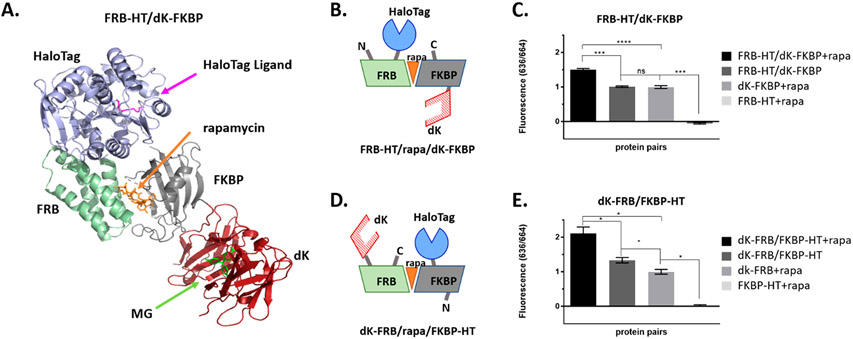
Figure 3.
FAP and HaloTag fusions to FRB and FKBP, induced to dimerize with rapamycin, show increased FAP–DAPA fluorescence, indicating proximity dependent activation. (A) Illustration of FRB-HT and FKBP-dK interacting with rapamycin created in silico from solved crystal structures (PDB: 4FAP, 5UXZ, and 4K3G). (B, D) The cartoons of selected fusions for this study showing pairs where the HT/FAP are on cis or trans faces of the FRB/rapa/FKBP trimer. (C, E) Fluorescence of rapamycin induced dimerization of FKBP and FRB fusions of dK and HT were measured at 100 nM dK fusion, 200 nM HT fusion, 250 nM rapamycin, and 10 nM DAPA. Addition of rapamycin to solutions containing FRB/FKBP fusions show significant increase in FAP–DAPA signal when compared to both proteins in absence of rapamycin or dK fusions alone (unpaired, two sided, t test, *p < 0.05; ****p ≤ 0.0001, ***0.0001 < p ≤ 0.001, **0.0001 < p ≤ 0.01, *p ≤ 0.05).