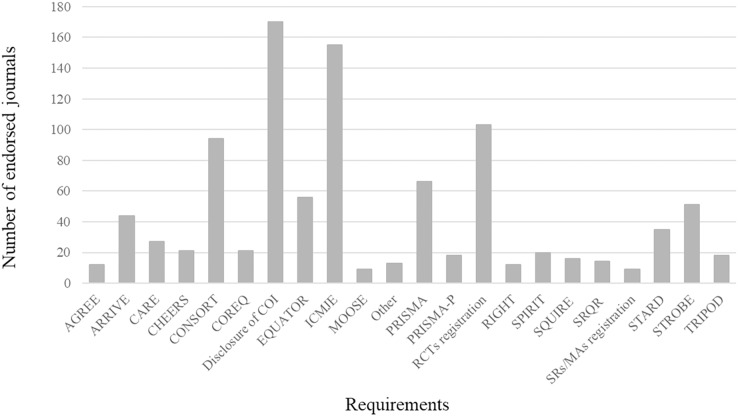
Figure 3. Frequency of endorsement of each requirement in included surgery journals (n = 188).
AGREE, Appraisal of Guidelines for Research and Evaluation; ARRIVE, Animal Research: Reporting of In Vivo Experiments; CARE, CAse REports; CHEERS, Consolidated Health Economic Evaluation Reporting Standards; CONSORT, Consolidated Standards of Reporting Trials; COREQ, Consolidated criteria for reporting qualitative research; COI, Conflict of Interest; EQUATOR, Enhancing the Quality and Transparency Of health Research; ICMJE, International Committee of Medical Journal Editors; MOOSE, Meta-Analysis Of Observational Studies in Epidemiology; Other, The minority percentage of reporting guidelines; PRISMA, Preferred Reporting Items for Systematic reviews and Meta-Analyses; PRISMA-P, Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols; RCTs, Randomized Controlled Trials; RIGHT, Reporting Items for Practice Guidelines in Healthcare; SPIRIT, Standard Protocol Items for Reporting in Trials; SQUIRE, Standards for Quality Improvement Reporting Excellence; SRQR, Standards for Reporting Qualitative Research; SRs/MAs, Systematic Reviews/Meta Analyses; STARD, Standards for Reporting Diagnostic accuracy; STROBE, Strengthening the Reporting of Observational studies in Epidemiology; TRIPOD, Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis.