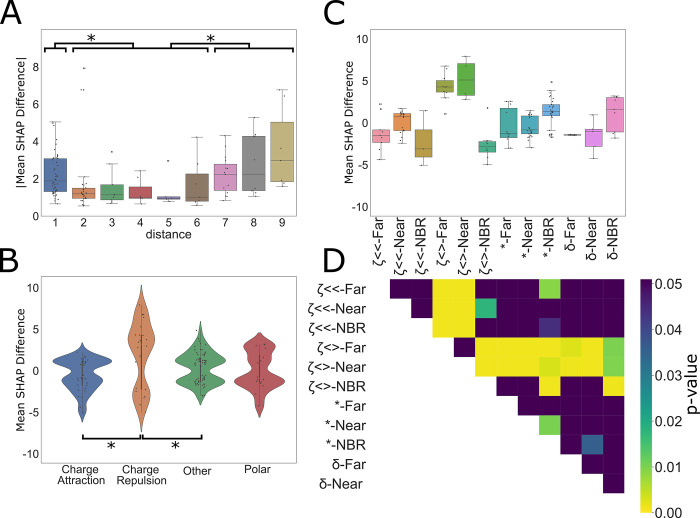
Fig 7. Dependence analysis of CCS model.
(A) Significant (Bonferroni corr. P-value < 0.05) values were taken from the interpositional dependence analysis and the difference in the mean between the interdependent amino acids SHAP values and the remaining amino acids at each compared position pair were grouped based on the distance between the dependent interaction, (B) the category of interaction, or (C) distance and interaction category. Categories are labelled by the following for the combined bar plot and heatmap: ζ<< = charge attraction, ζ<> = charge repulsion, * = other, and δ = polar. For the distance analysis, interactions were grouped into three categories, neighboring (distance = 1), near (distance = 2, 3, 4, 5,6), and far (distance = 7, 8, 9). * indicates significance (ANOVA with Tukey’s post hoc test p-value < 0.05). For the interaction categories in (B) and (C), each interaction was grouped by the expected type of interaction between the two amino acids. Significant differences between interaction types are noted by the pairing by lines (ANOVA with Tukey’s post hoc test p-value < 0.05). (D) Significant differences between combined categories are illustrated by the heatmap where significant values (ANOVA with Tukey’s post hoc test p-value < 0.05) are designated by colors other than purple. Exact p-values for each are provided in S4 Table. repulsive molecular interactions, including charge repulsion and “other” interactions (likely steric interactions or interactions between the termini) increased predicted CCS. Notably, there were very few significant hydrophobic interactions. This may reflect that hydrophobic interactions between amino acids in a peptide act to minimize contact with a polar solvent, rather than acting as an attractive force itself. Peptides lose polar solvent (water) during the electrospray process, which may prevent significant hydrophobic interactions, which might contradict prior work [64].