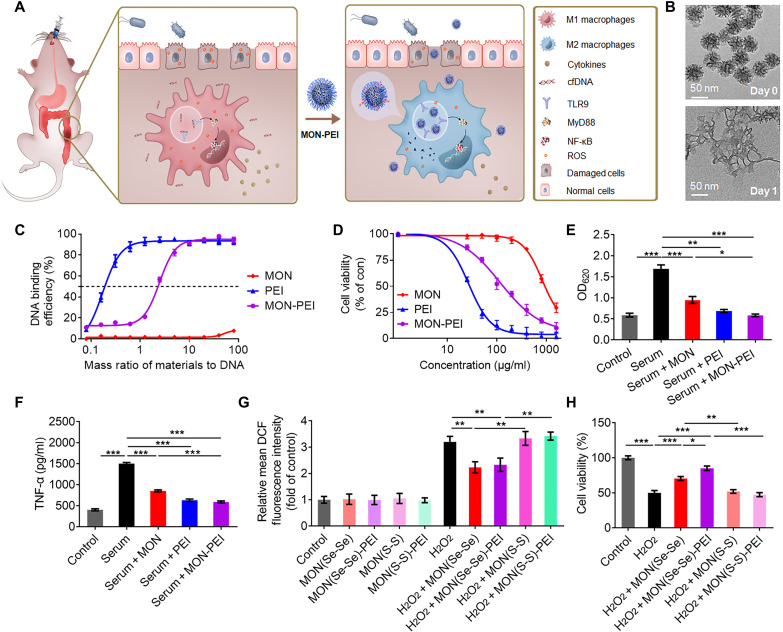
Fig. 2. MON-PEI reduces cfDNA- and ROS-induced inflammation in vitro.
(A) Schematic of the design of a biodegradable nanomedicine with cfDNA- and ROS-scavenging activity for IBD therapy. (B) Transmission electron microscopy images of MON before and after a 1-day incubation in simulated body fluid solution containing 100 μM H2O2. (C) DNA binding efficiency of MON, PEI, and MON-PEI at different nanoparticle:DNA mass ratios at 37°C. (D) Viability of Caco-2 cells treated for 24 hours with various concentrations of MON, PEI, and MON-PEI. (E) Activation of HEK-TLR9 reporter cells by IBD patient sera in the absence or presence of MON, PEI, and MON-PEI for 24 hours. The corresponding SEAP activity in supernatants from each group was determined with a QUANTI-Blue assay at OD620. (F) RAW 264.7 macrophages were stimulated with IBD patient sera in the absence or presence of MON, PEI, and MON-PEI for 24 hours. Supernatants were assayed for TNF-α by ELISA. (G) Relative fluorescence intensity of oxidized DCF in Caco-2 cells after incubation with different formulations in the presence or absence of 100 μM H2O2 for 4 hours. (H) The viability of Caco-2 cells was measured after treatment with different formulations in the presence of 100 μM H2O2 for 24 hours. Data are means ± SEM (n = 3 independent experiments; *P < 0.05, **P < 0.01, and ***P < 0.001 by one-way ANOVA with Tukey’s multiple comparison test).