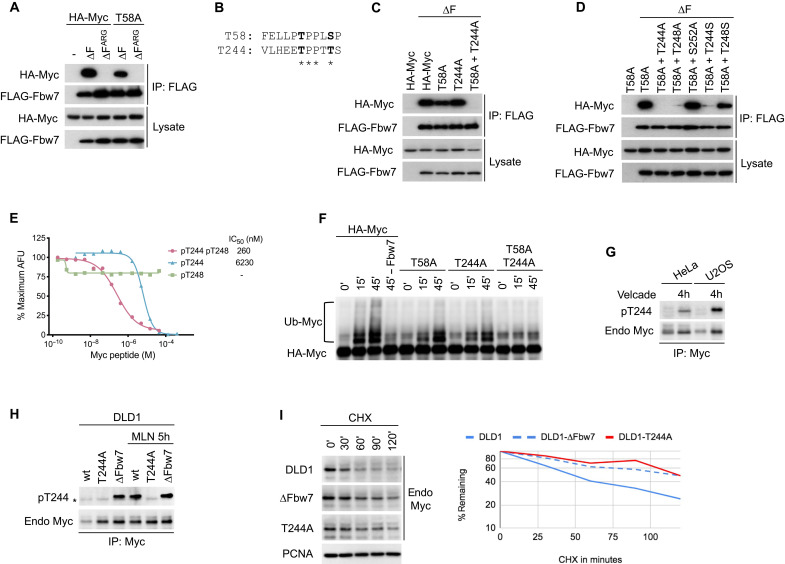
Fig. 1. Identification of the T244 degron.
(A) HEK293 cells were cotransfected with HA-Myc and FLAG-Fbw7, and lysates were immunoprecipitated (IP) with FLAG antibody and blotted with HA antibody. F-box deletion (∆F) uncouples binding from turnover. (B) Alignment of the T58 and T244 degrons. (C and D) Assays as in (A). To isolate the T244 degron, all mutants were made in a T58A mutant background in (D). S252 was tested for its potential role as a priming site for degron phosphorylations. (E) Quantitative peptide competition analysis of Myc peptides with a cyclin E pT380/pS384 degron peptide for Fbw7 binding as measured in arbitrary fluorescent units (AFU). (F) Myc or mutants were immunoprecipitated from transfected HEK293 cell lysates, and washed beads were subjected to in vitro ubiquitylation. Fbw7 was omitted in lane 4 (−Fbw7) as negative control. Reactions were analyzed by Western blotting. (G) HeLa and U2OS cell lines were treated with proteasome inhibitor Velcade for 4 hours (lanes 2 and 4), and lysates were immunoprecipitated against Myc and blotted as indicated. While Myc levels increase upon proteasome inhibition, pT244 appears to be increased relative to total Myc levels. (H) Isogenic DLD1 WT cells or their homozygously mutated cell lines (T244A or Fbw7 deletion) were treated with MLN4924 (right lanes only), and lysates were immunoprecipitated against Myc and blotted as indicated. Asterisk marks an antibody cross-reaction of the IP antibody heavy chain. (I) DLD1 cells or isogenic derivatives homozygously deleted for Fbw7 or T244A Myc mutated were treated with cycloheximide (CHX) and blotted for Myc steady-state abundance. Proliferating cell nuclear antigen (PCNA) serves as loading control. Bands were quantitated and plotted on the right.