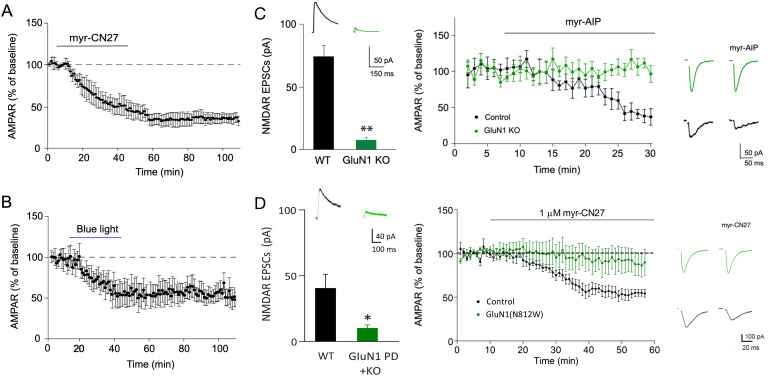
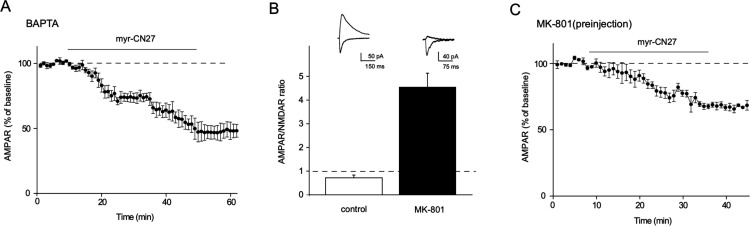
Figure 4. The depression caused by transient inhibition of Ca2+-calmodulin-dependent kinase II (CaMKII) is long lasting and is absent in cells lacking NMDARs.
(A) In acute slices, time course showing that following myr-CN27 application AMPAR synaptic transmission remains depressed (the difference between before and after myr-CN27: n = 4, p < 0.01, two-tailed Wilcoxon Signed Rank Test). (B) In culture slices, time course showing that following blue light exposure on paAIP2 expressing cells AMPAR synaptic transmission remains depressed (the difference between before and after blue light exposure: n = 5, p < 0.01, two-tailed Wilcoxon Signed Rank Test). (C) Left panel, paired recordings from acute slices of control cells and those expressing Cre in GluN1 floxed mice (n = 5, p < 0.01, two-tailed Wilcoxon Signed Rank Test). Middle panel, time course of the myr-AIP effect on AMPAR synaptic transmission in control cells (n = 5) and interleaved cells expressing Cre in GluN1 floxed mice (n = 5). p < 0.01, Mann–Whitney U test. Right panel, sample traces showing myr-AIP inhibited AMPAR synaptic transmission in control cell (black traces), but not in a GluN1KO cell (green traces). Mean ± standard error of the mean (SEM). (D) Left panel, paired recordings from acute slices of control cells and those expressing Cre and N812W (pore dead, PD) in GluN1 floxed mice (n = 6, p < 0.05, two-tailed Wilcoxon Signed Rank Test). Middle panel, the action of myr-CN27 is not rescued by expressing a GluN1 pore dead mutant (n = 8). Right panel, sample traces showing myr-CN27 inhibited AMPAR synaptic transmission in control cell (black traces), but not in a GluN1KO + PD cell (green traces).