Abstract
Background
Telomeres are essential for chromosomal stability and may play a key role in carcinogenesis. Telomere length is suggested as a tentative biomarker of risk for renal cell carcinoma (RCC). However, results of previous association studies between telomere length and risk for RCC are inconsistent.
Methods
We evaluated RCC risk in relation to peripheral blood leukocyte telomere length using a hospital-based case–control study of 169 RCC cases and 189 controls. Cases were histologically-confirmed RCC patients who were treated at the Moffitt Cancer Center (Tampa, FL). Controls with no history of cancer underwent a screening exam at the Lifetime Cancer Screening Center at Moffitt Cancer Center to rule out the presence of cancer. Relative telomere length (RTL) was measured by quantitative real-time polymerase chain reaction (PCR) using peripheral blood leukocyte DNA. Logistic regression was used to determine the association between RTL and RCC risk.
Results
As expected, increasing age was inversely correlated with RTL (Pearson r=−0.213, P=0.003) among controls but not cases. Average RTL was significantly shorter in cases as compared with controls [mean ± standard deviation (SD): 3.18±1.50 and 4.39±1.99, respectively, P<0.001]. In contrast, average RTL was not significantly different by gender, race, smoking status among controls or by clinical stages among RCC cases. In regression analysis, we observed that shorter RTL is significantly associated with RCC risk [odds ratio (OR) =1.48; 95% confidence interval (CI): 1.27–1.71] after adjustment for covariates.
Conclusions
We found that shorter RTL is associated with an increased risk for RCC. Our findings suggest that telomere length may be involved in the development of RCC.
Keywords: Telomere length, renal cancer, risk
Introduction
Renal cell carcinoma (RCC) accounts for 3.8% of all new cancer cases in US and its incidence rate has been increased over the last several decades (1). Although a few clinical tests, GFR (glomerular filtration rate) and creatinine level, are available for predicting renal function, the typical detection methods for RCC are abdominal imaging, such as ultrasonography, magnetic resonance imaging and computed tomography, then confirmed by histological evaluation (2). Similar to other cancers, early detection of RCC is a significant challenge. Therefore, identification of biomarkers is critical to improve current management of RCC.
Telomeres maintain the structural stability of chromosomes by preventing aberrant recombination during cell division and fusion of chromosomal ends (3). In general, telomeres shorten with each cell division, and eventually chromosomal abnormalities can occur if telomere length (TL) becomes critically short, which leads to cellular apoptosis or senescence (4-6). The role of telomeres in carcinogenesis is not well established in RCC.
The association between TL and cancer risk is also inconsistent across different malignancies (6,7). Several studies have reported that TLs are negatively associated with risk for various cancers, including breast, stomach, lung and myelodysplastic syndrome (MDS) (7,8). Meanwhile, other investigators have observed significant associations between long TL and increased risk of melanoma, breast, lung, and pancreatic cancers (9-14). Likewise, several RCC studies have reported inconsistent results. Two case-control studies reported no association between TL in leukocytes and risk for RCC (15,16). However, two earlier small studies (17,18) reported that individuals with short telomeres had a significantly increased risk of RCC. In addition, three studies evaluated the role of TL in survival and reported conflicting results. Svenson et al. [2009] reported an association between long TL and poor survival (19) while two studies reported that short TL was associated with poor prognosis for RCC (20,21). Pal et al. (22) reported that TL was significantly shorter in renal tumor tissues compared to that of paired adjacent renal tissues. In the present study, we aimed to investigate the differential association between TL and RCC risk in a hospital-based case-control study. We further sought to investigate the associations between TL and patient characteristics to glean insights into how telomeres may influence RCC risk.
Methods
Study population
RCC cases comprised of patients undergoing a surgical treatment for a suspected RCC under the care of the Genitourinary Oncology (GU) Program at Moffitt Cancer Center in Tampa, FL and enrolled between 2011 and 2014 in Moffitt’s RCC Study Cohort. This cohort protocol aims to enroll all Moffitt surgical RCC patients, obtaining biospecimens, clinical data and questionnaire information that were accessed. Cases in the current study did not have a previous cancer history and provided a blood sample before treatment within 3 months of a confirmed renal cancer diagnosis.
Controls were recruited from genitourinary (GU) Clinics or from the Lifetime Cancer Screening Center at the Moffitt Cancer Center. Briefly, the controls are individuals who visited the GU clinic or underwent a routine cancer screening exam at Moffitt’s Lifetime Cancer Screening Center between 2011 and 2014, were found to be cancer-free upon physical examination, and self-reported no history of any cancer. At the time of enrollment, controls provided blood samples and completed a structured questionnaire. The questionnaires were used to collect data on demographic characteristics and cancer risk factors including smoking status. Information for clinical and histologic characteristics of RCC cases, including histologic subtype, was obtained from the Moffitt Cancer Registry and patient medical records. All patients provided a written informed consent and this study protocol was approved by the University of South Florida (USF) Institutional Review Board.
Telomere length measurement
Relative telomere length (RTL) was measured in peripheral blood leukocytes from blood samples, obtained from cases before treatment, within three months of pathologic confirmation. Genomic DNA samples were extracted from leukocytes using the FlexiGene DNA kit (Qiagen), then stored at −20 °C until use. The RTL was measured using Cawthon’s real time PCR method as we reported previously (8,9,23,24). Briefly, amplification of genomic DNA from blood samples and reference DNA were performed using two different primer set with the T primer pair (tel1, 5'-GGTTTTTGAGGGTGAGGGTGAGGGTGAGGGTGAGGGT-3'; tel2, 5'-CCCGACTATCCCTATCCCTATCCCTATCCCTATCCCTA-3') and the S primer pair (36B4u, 5'-CAGCAAGTGGGAAGGTGTAATCC-3'; 36B4d, 5'-CCCATTCTATCATCAACGGGTACAA-3'). Reference DNA from the T47D cell line was used for a standard curve with five different concentrations. Quantitative real time PCR was performed with the following cycling profiles: for telomere amplification: 95 °C for 10 min followed by 30 cycles of 95 °C for 15 s and 54 °C for 2 min; for 36B4: 95 °C for 10 min, 40 cycles of 95 °C for 15 s and 60 °C for 1 min.
The ratio of telomere (T) repeat copy number to a single-copy gene (S) copy number (T/S ratio) for each sample was estimated by subtracting the mean threshold cycle value (Ct) for 36B4 from the mean telomere Ct. The single copy gene used as a reference was 36B4. The relative T/S ratio was estimated by subtracting the T/S ratio of the standard curve point from the T/S ratio of each unknown sample. Telomere and 36B4 amplifications were performed in triplicate for each participant. The means of triplicates were used for further analysis. In addition, 5 internal replicated quality control samples were included in all 386 well plates to assess inter and intra assay variability.
Statistical analysis
The participants’ demographic and clinical factors by the RCC status were compared using the Student’s t-test. Pearson correlation test was performed to confirm an inverse correlation between age and RTL and validity of RTL assay. Linear regression was used to test for differences in RTL by age, gender, race and smoking status. Logistic regression was used to test for the association between TL and RCC risk with and without adjusted for the above covariates. RTL and age were used as continuous variables, while gender, race and smoking status were used as categorical variables. All P values were two-sided and considered statistically significant at P value <0.05. SAS version 9.1.2 (Cary, NC, USA) was used to conduct all analyses.
Results
Table 1 presents selective descriptive characteristics of the RCC cases and controls and distribution of RTLs by different variables and the RCC status. Compared to controls, RCC cases were more likely to be male, older, and ever-smokers. The mean age of controls was 55.7 years and 61.3 years among RCC cases (P<0.001).
Table 1. Selected characteristics of and relative telomere length (RTL) by RCC cases & controls.
| Characteristics | Controls (n=189) | Renal cases (n=169) | ||||
|---|---|---|---|---|---|---|
| RTL1 | N (%) | RTL1 | N (%) | P value | ||
| RTL | 4.39±1.99 | 189 (100.0) | 3.18±1.50 | 169 (100.0) | <0.001 | |
| Age2 | 55.7±10.0 | 61.3±12.2 | <0.001 | |||
| Gender | ||||||
| Female | 4.22±1.65 | 94 (49.7) | 2.99±1.27 | 70 (41.4) | 0.0001 | |
| Male | 4.55±2.28 | 95 (50.3) | 3.33±1.64 | 99 (58.6) | 0.0001 | |
| Race | ||||||
| White | 4.29±1.91 | 107 (56.6) | 3.24±1.53 | 140 (82.8) | <0.0001 | |
| Black | 4.29±2.33 | 25 (13.2) | 2.83±0.97 | 16 (9.5) | 0.02 | |
| Latino | 4.62±2.04 | 54 (28.6) | 3.05±1.92 | 9 (5.3) | 0.04 | |
| Other | 4.51±1.31 | 3 (1.60) | 2.87±1.36 | 4 (2.4) | 0.17 | |
| Smoking status | ||||||
| Never | 4.51±2.12 | 96 (50.8) | 3.24±1.70 | 69 (40.8) | <0.0001 | |
| Ever | 4.26±1.92 | 93 (49.2) | 3.14±1.36 | 100 (59.2) | <0.0001 | |
| Histology | − | − | 0.38 | |||
| Clear cell RCC | 3.10±1.56 | 105 (62.1) | ||||
| Papillary RCC | 3.54±1.53 | 30 (17.8) | ||||
| Chromophobe RCC | 3.21±1.17 | 11 (6.5) | ||||
| RCC unspecified | 3.27±1.40 | 19 (11.2) | ||||
| Other RCCs | 2.09±0.28 | 4 (2.4) | ||||
| AJCC stage | − | − | 0.45 | |||
| I & II | 3.22±1.55 | 137 (81.1) | ||||
| III & IV | 3.00±1.30 | 32 (18.9) | ||||
1, data are shown as mean ± standard deviation, P value based on t-tests by comparing RTL for each selected sub-groups between cases and controls. 2, age at diagnosis (case) or at consent (control). RCC, renal cell carcinoma; AJCC, American Joint Committee on Cancer.
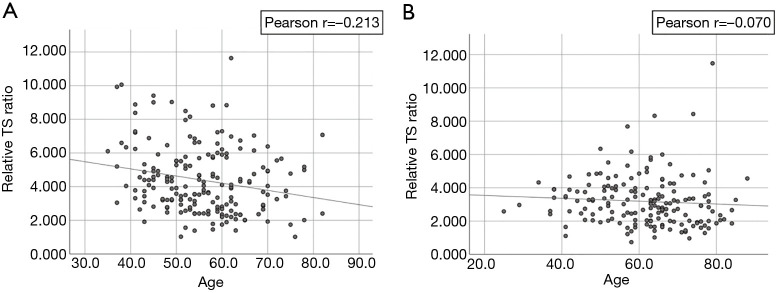
RTL was inversely correlated with age (Pearson r=−0.213, P=0.003) (Figure 1) among controls but not cases (Pearson r=−0.070, P=0.363). RTL did not differ significantly by gender, race and smoking status in both groups (Table 2). Among the RCC case, we did not observe an association between RTL and AJCC clinical stage (P=0.45 stage I & II vs. stage III & IV) or histological types (P=0.38). Clear cell RCC was the most common histological subtype (62.1%, 105/169) and the majority of cases had stage I disease (75.7%, 128/169). The ranges of RTL in controls and cases are similar (1.02–11.6 and, 0.73–11.48). However, mean RTL was significantly shorter among cases compared with controls (mean ± standard deviation: 3.18±1.50 vs. 4.39±1.99, respectively, P<0.001) (Table 1, Figure 2).
Figure 1.
Relationship of age and the relative telomere length. (A) Relationship between age and the telomere length among control; as the age increases, the telomere length decreases in control; (B) relationship between age and telomere length among case, an inverse relationship between age and telomere length was not observed among cases.
Table 2. Variables correlated with relative telomere length (RTL).
| Variables | Univariable model coefficient | P value | Multivariable model coefficient | P value |
|---|---|---|---|---|
| Controls | ||||
| Age | −0.213 | 0.003 | −0.219 | 0.003 |
| Smoking (ever vs. never) | −0.063 | 0.391 | −0.063 | 0.405 |
| Gender (female vs. male) | −0.082 | 0.264 | −0.128 | 0.097 |
| Race (non-white vs. white) | 0.008 | 0.908 | 0.073 | 0.336 |
| Cases | ||||
| Age | −0.070 | 0.363 | −0.088 | 0.261 |
| Smoking (ever vs. never) | −0.030 | 0.695 | −0.080 | 0.322 |
| Gender (female vs. male) | −0.116 | 0.135 | −0.140 | 0.082 |
| Race (non-white vs. white) | 0.085 | 0.271 | 0.104 | 0.182 |
Figure 2.
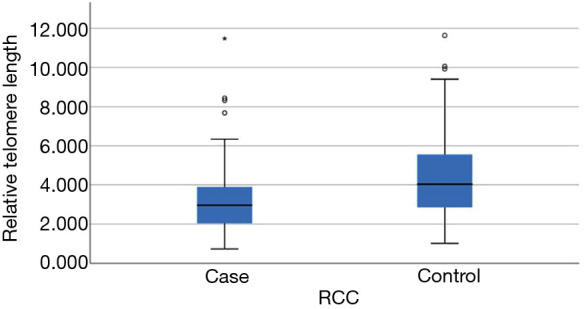
Relative telomere lengths in renal cell carcinoma (RCC) cases and controls. Relationship between RCC status and the relative telomere length (RTL). This boxplot showed range, 25 and 75 percentiles, and median of RTL in RCC patients and controls. RTL was significantly higher in the control than the RCC group (*, P<0.0001).
We also found that shorter RTL was significantly associated with increased RCC risk (OR=1.52, 95% CI: 1.32–1.75) using the logistic regression (Table 3). Age is an independent risk factor for RCC (OR=1.05, 95% CI: 1.03–1.07); however, gender (P=0.06), race (P=0.09) and smoking status (P=0.12) were not significantly associated with RCC risk. The association between shorter RTL and RCC risk remained significant after adjusting gender, race, age and smoking status (OR=1.48, 95% CI: 1.27–1.71).
Table 3. Variables associated with renal cell carcinoma (RCC) risk.
| Variables | Crude OR (95% CI) | Adjusted* OR (95% CI) |
|---|---|---|
| Age | 1.05 (1.03–1.07) | 1.03 (1.01–1.06) |
| Smoking | 1.50 (0.98–2.27) | 1.21 (0.75–1.94) |
| Gender (male/female) | 1.40 (0.92–2.13) | 1.55 (0.96–2.51) |
| Race | 0.93 (0.85–1.01) | 0.93 (0.84–1.02) |
| Relative telomere length | 1.52 (1.32–1.75) | 1.48 (1.27–1.71) |
*, the model included gender, age, race, smoking status and relative telomere length (RTL). OR, odds ratio; CI, confidence interval.
Discussion
In this study, we found that short RTL was associated with an increase in risk for RCC using a hospital-based case-control study design. Telomere shortening causes chromosomal instability, eventually may lead to carcinogenesis. The classic study on dyskeratosis congenita (DC) reported that individuals with defective telomere process have 11-fold higher risk of cancer compared with the general population (25). Further, shorter RTL was observed in peripheral blood leukocytes in many cancer cases. Therefore, individuals with shorter RTL may have a higher risk of cancer. Our findings are consistent with results from previous studies (17,18) in which both suggested that shorter telomeres were associated with an increased risk for RCC. In contrast to the results from the current study, the same associations were not confirmed in other previous studies (15,16). A recent large RCC study reported that a longer RTL is strongly associated with increased RCC risk (26). However, this study used genetically-predicted TL based on genetic variants, which are associated with longer RTL. Thus, rather than direct measuring TL from blood samples, genetic polymorphisms as a surrogate of RTL were used to evaluate the relationship with RCC risk (26).
These inconsistent results among studies may be contributed by differences distribution of risk factors associated with RTL, such as age, smoking and chronic diseases (i.e., diabetes, and hypertension) across study populations. Furthermore, different laboratory procedures for measuring TL, storage condition of blood samples, and DNA extraction methods were suggested as potential sources underlying inconsistent results on association studies between cancer risks and TL (27).
The strength of this study is a strong internal validity because all participants were from a single hospital. Thus, they were managed in a relatively consistent manner and clinical information were gathered in a similar fashion. Further, the blood samples from the RCC patients were collected before treatments within three months of a confirmed renal cancer diagnosis. Therefore, we do not anticipate a potential confounding effect from treatments, which might influence telomere biology process. Limitations of the current study include the use of hospital-based controls, which limits generalizability to a larger community-based population. However, the patient and control populations, who were served by the Moffitt Cancer Center, and Moffitt affiliated Lifetime Cancer Screening Center, are from similar catchment areas. Therefore, selection bias not likely exists because both groups had similar environmental exposures. Any difference in telomere length between cases and control may be due to exposure to risk factors of RCC that also influence TL. Furthermore, we measured RTL from patients’ blood samples collected prior to the treatment. Therefore, treatment effect on RTL was not anticipated. However, because RTL was measured after diagnosis of RCC, these RTL changes in blood may be a consequence of renal tumor (i.e., reverse causation) rather than an etiologic biomarker. In addition, TL in peripheral blood samples may not represent TL in renal tissues. Although we controlled for age variable in logistic regression, some residual confounding effect still may exist. Additional limitations include lack of data on other factors that influence telomere length and RCC risk, such as smoking, alcohol, obesity, personal history of diabetes, hypertension and germline variation in telomere maintenance genes, which may have influenced the results to some extent.
In summary, our data suggested that a short TL in blood is associated with increased RCC risk. Our results provide additional information that TL could be an integrative molecular marker of RCC and could have value for early detection of RCC toward timely clinical intervention. Given the small sample size and inconsistent results with previous studies, additional studies are needed to further investigate the association between TL and RCC in larger cohorts and examine the effect of TL on prognosis of RCC.
Acknowledgments
Funding: The development of this manuscript was supported by Florida State James Esther King biomedical program and R01CA134466 (PI: Parker) and DeBartolo Family Personalized Medicine Institute (PI: Park).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All patients provided a written informed consent and this study protocol was approved by the University of South Florida (USF) Institutional Review Board.
Footnotes
Provenance and Peer Review: This article was commissioned by the Guest Editors (Hui-Yi Lin, Tung-Sung Tseng) for the series “Population Science in Cancer” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.06.36). The series “Population Science in Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 2.van Oostenbrugge TJ, Futterer JJ, Mulders PFA. Diagnostic Imaging for Solid Renal Tumors: A Pictorial Review. Kidney Cancer 2018;2:79-93. 10.3233/KCA-180028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deng Y, Chang S. Role of telomeres and telomerase in genomic instability, senescence and cancer. Lab Invest 2007;87:1071-6. 10.1038/labinvest.3700673 [DOI] [PubMed] [Google Scholar]
- 4.Mooi WJ, Peeper DS. Oncogene-induced cell senescence--halting on the road to cancer. N Engl J Med 2006;355:1037-46. 10.1056/NEJMra062285 [DOI] [PubMed] [Google Scholar]
- 5.Londono-Vallejo JA. Telomere length heterogeneity and chromosome instability. Cancer Lett 2004;212:135-44. 10.1016/j.canlet.2004.05.008 [DOI] [PubMed] [Google Scholar]
- 6.Antwi SO, Petersen GM. Leukocyte Telomere Length and Pancreatic Cancer Risk: Updated Epidemiologic Review. Pancreas 2018;47:265-71. 10.1097/MPA.0000000000000995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wentzensen IM, Mirabello L, Pfeiffer RM, et al. The association of telomere length and cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2011;20:1238-50. 10.1158/1055-9965.EPI-11-0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rollison DE, Epling-Burnette PK, Park JY, et al. Telomere length in myelodysplastic syndromes. Leuk Lymphoma 2011;52:1528-36. 10.3109/10428194.2011.568648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anic GM, Sondak VK, Messina JL, et al. Telomere length and risk of melanoma, squamous cell carcinoma, and basal cell carcinoma. Cancer Epidemiol 2013;37:434-9. 10.1016/j.canep.2013.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gramatges MM, Telli ML, Balise R, et al. Longer relative telomere length in blood from women with sporadic and familial breast cancer compared with healthy controls. Cancer Epidemiol Biomarkers Prev 2010;19:605-13. 10.1158/1055-9965.EPI-09-0896 [DOI] [PubMed] [Google Scholar]
- 11.Svenson U, Nordfjall K, Stegmayr B, et al. Breast cancer survival is associated with telomere length in peripheral blood cells. Cancer Res 2008;68:3618-23. 10.1158/0008-5472.CAN-07-6497 [DOI] [PubMed] [Google Scholar]
- 12.Shen M, Cawthon R, Rothman N, et al. A prospective study of telomere length measured by monochrome multiplex quantitative PCR and risk of lung cancer. Lung Cancer 2011;73:133-37. 10.1016/j.lungcan.2010.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lan Q, Cawthon R, Shen M, et al. A prospective study of telomere length measured by monochrome multiplex quantitative PCR and risk of non-Hodgkin lymphoma. Clin Cancer Res 2009;15:7429-33. 10.1158/1078-0432.CCR-09-0845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lynch SM, Major JM, Cawthon R, et al. A prospective analysis of telomere length and pancreatic cancer in the alpha-tocopherol beta-carotene cancer (ATBC) prevention study. Int J Cancer 2013;133:2672-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hofmann JN, Lan Q, Cawthon R, et al. A prospective study of leukocyte telomere length and risk of renal cell carcinoma. Cancer Epidemiol Biomarkers Prev 2013;22:997-1000. 10.1158/1055-9965.EPI-13-0142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofmann JN, Baccarelli A, Schwartz K, et al. Risk of renal cell carcinoma in relation to blood telomere length in a population-based case-control study. Br J Cancer 2011;105:1772-5. 10.1038/bjc.2011.444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu X, Amos CI, Zhu Y, et al. Telomere dysfunction: a potential cancer predisposition factor. J Natl Cancer Inst 2003;95:1211-8. 10.1093/jnci/djg011 [DOI] [PubMed] [Google Scholar]
- 18.Shao L, Wood CG, Zhang D, et al. Telomere dysfunction in peripheral lymphocytes as a potential predisposition factor for renal cancer. J Urol 2007;178:1492-6. 10.1016/j.juro.2007.05.112 [DOI] [PubMed] [Google Scholar]
- 19.Svenson U, Ljungberg B, Roos G. Telomere length in peripheral blood predicts survival in clear cell renal cell carcinoma. Cancer Res 2009;69:2896-901. 10.1158/0008-5472.CAN-08-3513 [DOI] [PubMed] [Google Scholar]
- 20.Weischer M, Nordestgaard BG, Cawthon RM, et al. Short telomere length, cancer survival, and cancer risk in 47102 individuals. J Natl Cancer Inst 2013;105:459-68. 10.1093/jnci/djt016 [DOI] [PubMed] [Google Scholar]
- 21.Callahan CL, Schwartz K, Ruterbusch JJ, et al. Leukocyte telomere length and renal cell carcinoma survival in two studies. Br J Cancer 2017;117:752-5. 10.1038/bjc.2017.237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pal D, Sharma U, Khajuria R, et al. Augmented telomerase activity, reduced telomere length and the presence of alternative lengthening of telomere in renal cell carcinoma: plausible predictive and diagnostic markers. Gene 2015;562:145-51. 10.1016/j.gene.2015.02.079 [DOI] [PubMed] [Google Scholar]
- 23.Lengacher CA, Reich RR, Kip KE, et al. Influence of Mindfulness-Based Stress Reduction (MBSR) on Telomerase Activity in Women With Breast Cancer (BC). Biol Res Nurs 2014;16:438-47. 10.1177/1099800413519495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res 2002;30:e47. 10.1093/nar/30.10.e47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alter BP, Giri N, Savage SA, et al. Cancer in dyskeratosis congenita. Blood 2009;113:6549-57. 10.1182/blood-2008-12-192880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Machiela MJ, Hofmann JN, Carreras-Torres R, et al. Genetic Variants Related to Longer Telomere Length are Associated with Increased Risk of Renal Cell Carcinoma. Eur Urol 2017;72:747-54. 10.1016/j.eururo.2017.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cunningham JM, Johnson RA, Litzelman K, et al. Telomere length varies by DNA extraction method: implications for epidemiologic research. Cancer Epidemiol Biomarkers Prev 2013;22:2047-54. 10.1158/1055-9965.EPI-13-0409 [DOI] [PMC free article] [PubMed] [Google Scholar]