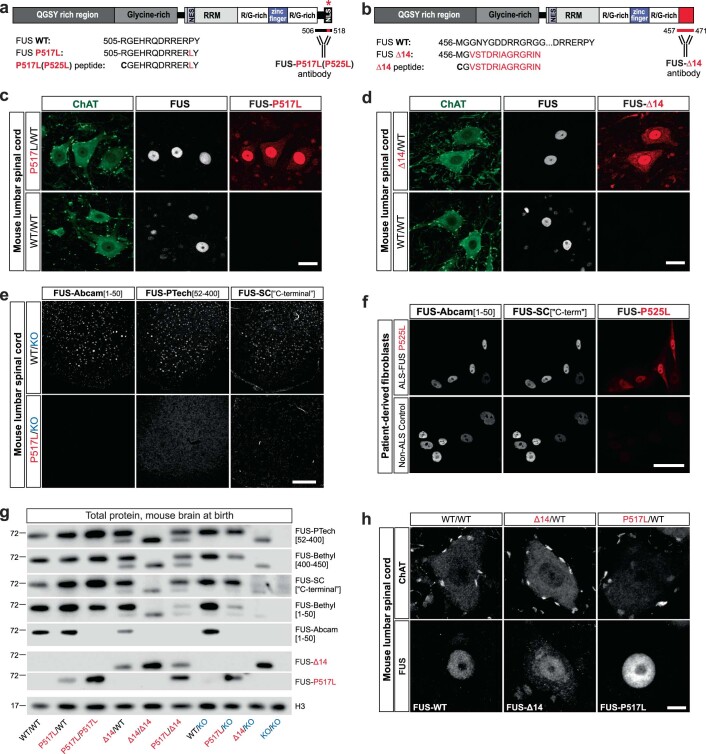
Extended Data Fig. 2. Variable reactivity of commercial antibodies with mutant FUS: characterization of mutant FUS expression and localization using novel mutant FUS-specific antibodies.
(a, b) Schematics of P517L and Δ14 mutant FUS proteins (top). Mutations are indicated by red asterisk for P517L and red C-terminal rectangle for Δ14. Bottom shows C-terminal amino acid sequences of FUS WT protein, FUS P517L and FUS Δ14 proteins, and the P517L and Δ14 synthetic peptides used for the generation of FUS-P517L and FUS-Δ14 antibodies (bottom). Red letters in the amino acid sequences indicate the residues specific to the FUS mutants. The locations of the epitopes for FUS-P517L and FUS-Δ14 antibodies are marked by horizontal bars below the C-termini of the corresponding schematized mutant proteins (bottom right). The murine and human C-terminal FUS amino acid sequences are conserved, and the illustrated mouse sequences also correspond to the human FUS P525L and Δ14 proteins. (c, d) Immunostaining of adult spinal cord sections of 1-year-old heterozygous mutant (P517L/WT and Δ14/WT) and WT/WT mice using FUS-Abcam[1–50] (FUS, white) and FUS-P517L or FUS-Δ14 (red) antibodies. Note the cytoplasmic staining with FUS-P517L andFUS-Δ14 antibodies in the animals that express the corresponding mutant FUS protein (upper panels) and the absence of staining of wild type FUS protein in WT/WT animals using either the P517L or the Δ14 antibodies (bottom panels). Scale bar: 20 µm. (e) Representative images of spinal cord sections from WT/KO and P517L/KO animals show lack of immunoreactivity of three commercial antibodies with P517L mutant FUS protein. Scale bar=100 µm. (f) Immunostaining of human cultured fibroblasts from an ALS-FUSP525L patient (top) and a non-ALS control (bottom) using two distinct FUSWT antibodies (white) and the FUS-P517L(P525L) antibody (red). Identical confocal microscopy settings (laser power and gain) were used for imaging the top and the bottom panels. Scale bar: 50 µm. (g) Denaturing immunoblot of brains of newborn mice of the indicated genotypes demonstrates variable affinities of five commercially available anti-FUS antibodies for mutant relative to wild type FUS. Note, for example, absence of visible bands for either P517L or Δ14 mutant FUS protein with the FUS-Abcam[1–50] antibody. In addition, absence of visible bands for WT protein confirms the specificity of FUS-P517L and FUS-Δ14 antibodies for mutant FUS. (h) Representative images of MNs from spinal cord sections of 2-year-old WT/WT (left), Δ14/WT (middle), and P517L/WT (right) animals stained with FUS-Abcam[1–50], FUS-Δ14, and FUS-P517L antibodies, respectively, show lack of obvious inclusions. Scale bar=5 µm.