Abstract
Background
Few studies have directly compared virus-specific antibodies and their neutralizing capacity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) wild type (WT) and circulating variants of concern despite the reported high efficacy of messenger RNA (mRNA)- and vector-based vaccines.
Objective
We assessed SARS-CoV-2 spike protein region 1 (S1)-specific antibodies of BNT162b2, mRNA-1273, and ChAdOx1 vaccinated as well as convalescent coronavirus disease 2019 (COVID-19) patients. We also determined the neutralization ability against SARS-CoV-2 WT and B.1.1.7 (Alpha), B1.1.7 E484K (Alpha-E484K), B.1.351 (Beta), and B.1.617.2 (Delta) variants.
Methods
Serum samples of 107 fully vaccinated or convalescent individuals were analyzed for anti–SARS-CoV-2-S1 IgG and IgA as well as for total anti–SARS-CoV-2 receptor binding domain Ig. Furthermore, neutralization capacity as 50% and 90% neutralization titer values against SARS-CoV-2 WT virus and circulating variants were determined.
Results
We observed a robust IgG response in all participants; however, the highest titers were detected in mRNA-based vaccine recipients. In case of serum IgA responses, the difference between mRNA- and vector-based vaccines or convalescent patients was even more pronounced. Interestingly, all 3 vaccines could neutralize all tested variants of concern in addition to WT virus, but in some individuals, only low or no neutralization, especially against Alpha-E484K and the Delta variant, was detected.
Conclusion
Our study of the efficacy of various COVID-19 vaccines found that mRNA-1273 had the highest neutralization abilities compared to BNT162b2 and ChAdOx1. COVID-19 convalescent patients demonstrated the most heterogeneous range of antibody titers and neutralization abilities, making it hard to assess protection. Furthermore, a significant positive relation between antibodies and the 50% neutralization titer values for immunized and convalescent individuals was determined.
Key words: SARS-CoV-2, variants of concern, vaccines, IgG and IgA antibodies, virus neutralization
Abbreviations used: Ab, Antibody; BAU, Binding antibody unit; BNT162b2, BioNTech/Pfizer COVID-19 vaccine; ChAdOx1, AstraZeneca COVID-19 vaccine; CI, Confidence interval; COVID-19, Coronavirus disease 19; mRNA, Messenger RNA; mRNA-1273, Moderna COVID-19 vaccine; NT50, 50% neutralizing titer; NT90, 90% neutralizing titer; RBD, Receptor binding domain; RoA, Ratio of absorbance; S1, Spike protein region 1; SARS-CoV-1/2, Severe acute respiratory syndrome coronavirus type 1/2; VOC, Variant of concern; WT, Wild type
The current coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is ongoing, causing a high burden of mortality and morbidity among the global population.1 In 2020, large-scale phase 3 clinical trials demonstrated the safety and efficacy of the first European Medicines Agency–approved vaccines, including BNT162b2 by BioNTech/Pfizer, mRNA-1273 by Moderna, and ChAdOx1 by AstraZeneca.2, 3, 4 Promising results from other candidate trials rapidly followed.5, 6, 7 The virus vector–based ChAdOx1 vaccine demonstrated 70.4% efficacy against symptomatic SARS-CoV-2 infections, while messenger RNA (mRNA)-based BNT162b2 and mRNA-1273 vaccine trials reported efficacies of 95% and 94.1%,2, 3, 4 which greatly exceeded the US Food and Drug Administration and European Medicines Agency requirements for approval, defined as 50% point estimate efficacy.8 , 9
As vaccines began to change the course of the pandemic, new SARS-CoV-2 variants emerged with the potential for higher transmission and more severe infection. These new virus variants acquired mutations primarily in the spike protein of the virus, especially in the receptor binding domain (RBD), which must be closely monitored. Changes in the RBD could potentially have disadvantages associated with reduction of antibody Ig binding and neutralization or treatment efficacy, increased transmissibility, disease severity, or diagnostic impact. According to the US Centers for Disease Control and Prevention, variants for which there is evidence of the aforementioned risks are defined as variants of concern (VOC) or variants of interest. Early in the pandemic, a variant carrying the D614G mutation rapidly became the dominant lineage in North America and Europe and was associated with an increased infectivity.10 In late 2020, a lineage emerging from the United Kingdom, defined as B.1.1.7 or the Alpha variant, with a N501Y mutation, was found to be more infectious and transmissible, but whether disease severity is affected remains unclear.11 , 12
Currently, the phylogenetic landscape has changed tremendously with the emergence of B.1.617.2, or the Delta variant, which has been demonstrated to be more transmissible and is suspected to increase disease severity.13 , 14 Concerns further arose after evidence was presented showing that emerging variants escape from SARS-CoV-2–specific antibodies.15 , 16 Recent studies highlighted a reduced neutralization of the Alpha variant and B.1.351, the Beta variant, by convalescent and BNT162b2 or ChAdOx1 vaccinated individual sera.15 , 16 Of note, only a few reports on mRNA-1273–induced neutralization on variants have been published and were mostly performed using pseudotyped viruses harboring mutant SARS-CoV-2 spike proteins, but not whole-virus isolates.17, 18, 19 Specific acquired RBD mutations, defined as E484K mutation, notably found in the two VOC B.1.351 and P.1 (Gamma variant), were associated with reduced neutralization capacity by vaccine-elicited sera.15 , 17 , 20 Cases of the Alpha variant’s carrying the escape mutation E484K were found first in the United Kingdom in February 2021. Because the Alpha variant was already associated with higher transmission, its possible combination with an escape mutation raised concerns, and it is still classified as a VOC by Public Health England.21 So far, the variant has not replaced other major variants; however, careful assessment of vaccine-induced as well as COVID-19–induced immunity has to be made as a result of the risk of causing local outbreaks.
We investigated humoral immune responses and virus neutralization abilities from COVID-19 recovered patients and vaccinated individuals. To this aim, we assessed IgG, IgA, and total SARS-CoV-2–specific antibody titers and furthermore determined the neutralization ability of COVID-19 vaccinated and recovered individuals against infectious clinical isolates of SARS-CoV-2. Vaccinated participants received 1 of the 3 vaccines approved in EU/EAA countries at the time of the study: BNT162b2 by BioNTech/Pfizer, mRNA-1273 by Moderna, or ChAdOx1 by AstraZeneca. Convalescent COVID-19 patients tested positive for SARS-CoV-2 wild type (WT) only. Serum was analyzed for anti–SARS-CoV-2 spike protein region 1 (S1) IgG and IgA as well as for total anti–SARS-CoV-2 RBD Ig. Furthermore, neutralization capacity as 50% neutralizing titer (NT50) as well as values from each vaccine group or COVID-19 recovered group was assessed against SARS-CoV-2 WT virus and the circulating variants B.1.1.7 (Alpha), B1.1.7 E484K (Alpha-E484K), B.1.351 (Beta), and B.1.617.2 (Delta).
Methods
Ethics statement
Written informed consent was obtained from all donors of leftover nasopharyngeal/oropharyngeal specimens and serum samples by the participating clinics. The ethics committee of the Medical University of Innsbruck approved the use of anonymized leftover specimens of COVID-19 patients (ECS1166/2020) and healthy donors (ECS1166/2018) for scientific purposes.
Human samples
In this study, 87 persons fully vaccinated with ChAdOx1 (n = 33), BNT162b2 (n = 31), or mRNA-1273 (n = 23) as well as 29 COVID-19 convalescent patients were included. The average age of all patients was 46 years (range, 22-86 years), and the percentage of female and male patients included in the study was 52.6% and 47.4%, respectively. The average sampling day of vaccinated persons after their second dose was day 39 (range, days 21-104). The average sampling day for COVID-19 convalescent patients after diagnosis with SARS-CoV-2 WT virus, defined as the time (days) between positive PCR testing and blood sampling, was 130 days (range, 22-206 days). All included COVID-19 cases were diagnosed as being of mild disease severity, which did not require any treatment or hospitalization. Detailed information of each individual from the different groups (ChAdOx1, BNT162b2, or mRNA-1273 vaccinated persons as well as convalescent patients) regarding age, sex, sampling days, and inclusion in neutralization experiments is presented in Table E1, Table E2, Table E3, Table E4.
Viruses
Clinical specimens for SARS-CoV-2 (Alpha-E484K and Delta) from COVID-19 positive swabs (ethics statement, ECS1166/2020) and SARS-CoV-2 viruses (WT, Alpha, and Beta) from repositories (BEI Resources, Manassas, Va; CFAR/NIBSC; nos. 52281, 54000, and 54009) were propagated according to the manufacturer’s instructions and used subsequently in neutralization assays.
Enzyme-linked immunosorbent assay
Serum and plasma samples from vaccinated or COVID-19 convalescent participants were retrieved from blood samples in serum or EDTA collection tube by centrifugation at 300 × g for 5 minutes, and serum or plasma fractions were carefully collected and stored at −80°C until use. Sera were analyzed by ELISA to assess SARS-CoV-2–specific Ig by 3 commercially available tests according to the manufacturer’s instructions: SARS-CoV-2 antibody (Ab) ELISA (WS-1096; Wantai Biological, Beijing, China), anti–SARS-CoV-2 QuantiVac ELISA IgG (EI 2606-9601-10 G; Euroimmun, Lübeck, Germany), and anti–SARS-CoV-2 ELISA IgA (EI 2606-9601 A; Euroimmun). SARS-CoV-2 Ab ELISA is a qualitative assay and detects all Ig against RBD and for the detection of anti–SARS-CoV-2 IgA and IgG against S1 protein the anti–SARS-CoV-2 ELISA IgA and the QuantiVac ELISA IgG were used. In order to obtain qualitative results, areas for positive (total Ig ≥ 1.1 ratio of absorbance [RoA]), borderline (1.1 > total Ig ≥ 0.9 RoA) or negative (total Ig < 0.9 RoA) results were defined according to the manufacturer’s instructions. For anti–SARS-CoV-2-S1, IgA values ≥ 1.1 RoA, 1.1 > IgA ≥ 0.8 RoA, and IgA < 0.8 RoA were defined as positive, borderline, and negative results, respectively. The QuantiVac ELISA IgG allows converting the relative units per milliliter to binding antibody unit per milliliter (BAU∙mL−1). In order to obtain qualitative results, relative unit values were recalculated to BAU∙mL−1 according to the manufacturer’s instructions; IgG ≥ 35.2 BAU∙mL−1 was considered a positive, 35.2 > IgG ≥ 25.6 BAU∙mL−1 a borderline, and IgG < 25.6 BAU∙mL−1 negative an anti–SARS-CoV-2-S1 IgG result.
Neutralization plaque assay
VeroE6/TMPRSS2 cells (1.2 × 105) were seeded in a 48-well plate with culture medium (Dulbecco modified Eagle high-glucose medium supplemented with 10% fetal calf serum, 1% l-glutamine, 1% penicillin/streptomycin; all reagents were obtained from Sigma-Aldrich, St Louis, Mo) and incubated overnight at 37°C and 5% CO2. The next day, whole serum or plasma samples were serial diluted from 1:8 to 1:1024 and incubated with SARS-CoV-2 WT or variant strains (4 × 102 PFU∙mL−1) for 1 hour at 37°C. After incubation, VeroE6/TMPRSS2 cells were inoculated with antibody-opsonized SARS-CoV-2 for 1 hour at 37°C and 5% CO2. After incubation, inoculate was replaced with culture medium containing 1.5% carboxymethylcellulose (Sigma-Aldrich). Cells were incubated for 3 days at 37°C and 5% CO2 before plaque visualization and counting. For this, cells were washed and fixed with 10% neutral buffered formalin (Sigma-Aldrich) for 1 hour at room temperature. Fixation was followed by staining using 0.5% (wt/vol) crystal violet solution (Sigma-Aldrich) for 15 minutes at room temperature. To determine the neutralizing capacity of NT50 and 90% neutralizing titer (NT90), values from neutralization curves were calculated by 4-parameter nonlinear regression in GraphPad Prism v9 software (GraphPad Software, La Jolla, Calif). In order to obtain qualitative results, NT50 ≥ 1:32, 1:32 > NT50 ≥ 1:16, and NT50 < 1:16 were set as a positive, borderline, or negative result. For NT90, areas were defined as follows: NT90 ≥ 1:16 positive, 1:16 > NT90 ≥ 1:8 borderline, and NT90 < 1:8 negative.
Statistical analysis
Statistical analysis was performed by GraphPad Prism. Statistical significance of SARS-CoV-2–specific antibody ratio as well as of NT50 and NT90 levels was determined by Mann-Whitney U test for nonparametric distribution.
Results
High SARS-CoV-2–specific antibody titers in vaccinated versus convalescent individuals
In this study, 83 fully vaccinated individuals immunized with either the vector-based vaccine ChAdOx1 (n = 33; see Table E1 in the Online Repository at www.jacionline.org) or 1 of the 2 mRNA-based vaccines BNT162b2 (n = 31; see Table E2 in the Online Repository) or mRNA-1273 (n = 19; see Table E3 in the Online Repository) as well as COVID-19 convalescent patients (n = 24, see Table E4 in the Online Repository) were included. The average sampling day of vaccinated persons after their second dose was day 41 (range, days 21-104), and the average sampling day for COVID-19 convalescent patients after positive PCR test was 137 days (range, days 22-206).
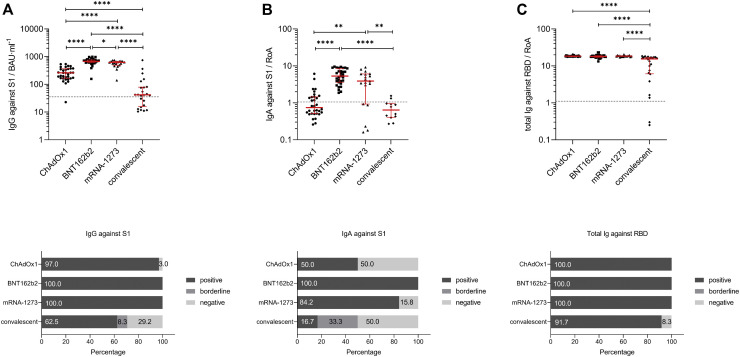
To characterize differences in the antibody titers induced by the 3 studied vaccines and in COVID-19 convalescent individuals, we first performed 3 SARS-CoV-2–specific antibody tests. Using ELISA-based test methods, we investigated the presence of SARS-CoV-2–specific IgG (Fig 1 , A, top) and IgA (Fig 1, B, top) antibodies against the S1 region of the SARS-CoV-2 spike protein in serum samples. In addition, total SARS-CoV-2–specific Ig (Fig 1, C, top) against the RBD of the viral spike protein were determined. These analyses revealed that 100% of individuals vaccinated with the mRNA vaccines BNT162b2 and mRNA-1273 tested positive for IgGs against the S1 region (Fig 1, A, bottom). In contrast, 97% of vector-based vaccine ChAdOx1 and only 62.5% patients of the convalescent group were positive for IgGs (Fig 1, A, bottom, ChAdOx1 and convalescent). Similar to IgGs, also 100% of BNT162b2 vaccinated participants were positive for serum IgA; respectively, 84.2%, 50%, and 16.7% tested positive for serum IgA in the mRNA-1273, ChAdOx1, and convalescent groups (Fig 1, B, bottom). Further analyses showed that median antibody titers of SARS-CoV-2–specific IgG and IgA were significantly higher in individuals vaccinated with mRNA-based vaccines such as BNT162b2 (IgG: 679.0; 95% confidence interval [CI] 626.1-733.7; IgA: 5.3; 95% CI, 3.9-7.1) and mRNA-1273 (IgG: 618.6; 95% CI, 492.4-672.9; IgA: 3.9; 95% CI, 0.9-6.0) compared to the vector-based vaccine ChAdOx1 (IgG: 259.5; 95% CI, 181.3-337.9; IgA: 0.7; 95% CI, 0.6-1.4) or detected in convalescent patients (IgG: 41.8; 95% CI, 17.6-77.6; IgA: 0.6; 95% CI, 0.4-1.0) (Fig 1, A and B, top; and see Table E5 in the Online Repository).
Fig 1.
SARS-CoV-2 antibody titer analysis using sera from fully vaccinated (ChAdOx1, BNT162b2, or mRNA-1273) or convalescent participants. A, Individual IgG antibody titer analysis against SARS-CoV-2-S1 domain for individuals fully vaccinated with ChAdOx1 (n = 33), BNT162b2 (n = 31), or mRNA-1273 (n = 23) and of convalescent patients (n = 29) were assessed with the anti–SARS-CoV-2 QuantiVac ELISA (IgG) kit. Results are presented as binding antibody unit per milliliter of serum (BAU∙mL−1). Corresponding percentages of participants and patients with positive, borderline, or negative IgG antibodies against SARS-CoV-2-S1 are visualized in the graph.B, Individual IgA antibody titer analysis against SARS-CoV-2-S1 domain for fully vaccinated and convalescent participants was performed with the anti–SARS-CoV-2-ELISA (IgA) kit. Results are presented as RoA. Corresponding percentages of participants with positive, borderline, or negative IgA antibodies against SARS-CoV-2-S1 are visualized in the graph.C, Individual total Ig antibody titer analysis against SARS-CoV-2 RBD for participants fully vaccinated and convalescent individuals was performed with the Wantai SARS-CoV-2 Ab ELISA kit. Results are presented as RoA. Corresponding percentages of participants with positive, borderline, or negative total Ig against SARS-CoV-2-S1 are visualized in the graph. Statistical significance between the 4 groups was determined by Mann-Whitney U test for nonparametric distribution (∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001). Medians are visualized as red lines together with the interquartile range as error bar. Cutoff values are illustrated by the gray dashed line.
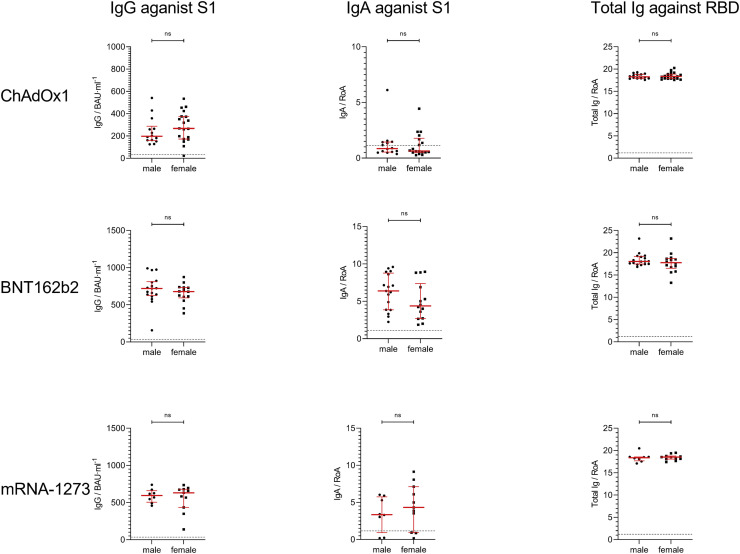
Significant differences were also found between the mRNA-based vaccines in induction of SARS-CoV-2–specific IgA, but not IgG, levels against the S1 region (Fig 1, A and B, top; BNT162b2 vs mRNA-1273). Analyses of total SARS-CoV-2–specific Ig against the RBD region of the spike protein demonstrated that 100% of vaccinated and 91.7% of convalescent COVID-19 patients tested positive (Fig 1, C, bottom). No significant differences were detected here between the 3 vaccinated groups (ChAdOx1: 18.4; 95% CI, 17.9-18.7; BNT162b2: 18.0; 95% CI, 17.5-18.7; mRNA-1273: 18.5; 95% CI, 18.1-18.7), but total Ig levels were significantly lower in convalescent (15.5; 95% CI, 6.4-17.0) compared to vaccinated individuals (Fig 1, C, top; Table E5). Furthermore, we investigated sex-specific differences for SARS-CoV-2–specific IgG, IgA, and total Ig induced in vaccinated and convalescent individuals, but no significant differences were found (see Fig E1 in this article’s Online Repository at www.jacionline.org).
Fig E1.
SARS-CoV-2 serum antibody titer comparison of female (F) and male (M) participants. Individuals fully vaccinated with ChAdOx1 (top row), BNT162b2 (middle row), and mRNA-1273 (bottom row). IgG antibody titer analysis against SARS-CoV-2-S1 domain for immunized participants who received ChAdOx1 (F = 19; M = 14), BNT162b2 (F = 14; M = 17), and mRNA-1273 (F = 14; M = 9) were performed with the anti–SARS-CoV-2 QuantiVac ELISA (IgG) kit. Results for IgG are presented in binding antibody unit per milliliter of serum (BAU∙mL−1). IgA antibody titers against SARS-CoV-2-S1 domain for vaccines were assessed with the anti–SARS-CoV-2 ELISA (IgA) kit. Results for IgA are presented as RoA. Total Ig antibody titer analysis against SARS-CoV-2 RBD immunized participant were obtained using the Wantai SARS-CoV-2 Ab ELISA kit. Results for total Ig are presented as RoA. Statistical significance was determined by Mann-Whitney U test for nonparametric distribution.
ChAdOx1-induced neutralization against SARS-CoV-2 and circulating variants
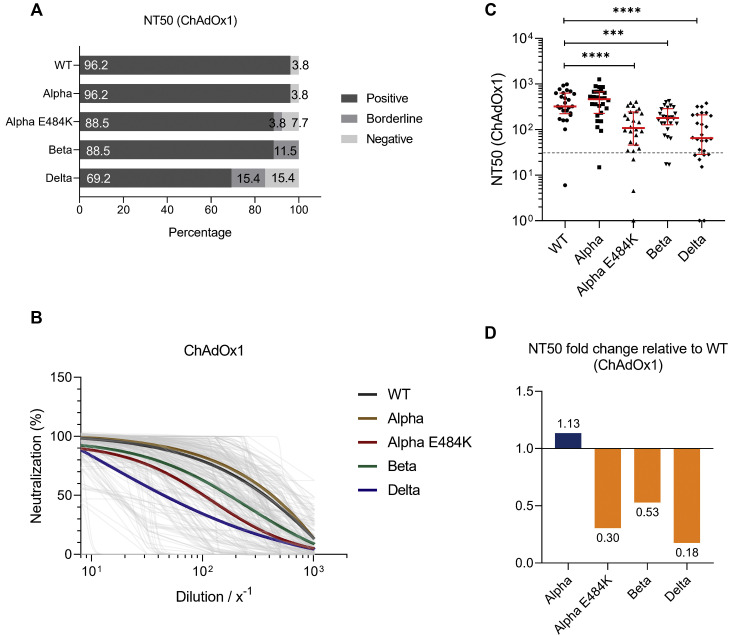
We then aimed to determine the neutralizing activity of individuals vaccinated with the vector-based vaccine ChAdOx1. In this regard, serum samples were incubated in various concentrations with the original virus strain and circulating variants that emerged since the end of 2020. Among these variants were SARS-CoV-2 Alpha, Beta, Alpha-E484K, and Delta.
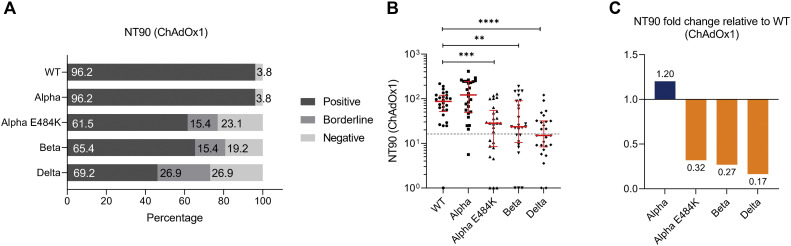
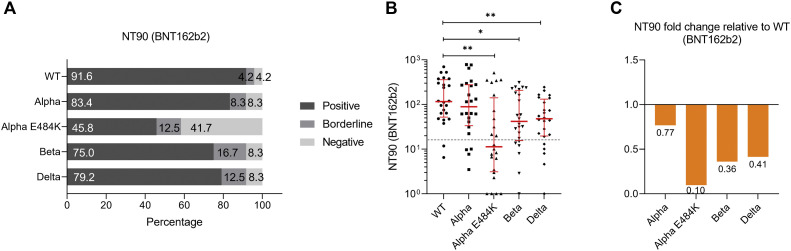
We calculated NT50 values and found that 96.2%, 96.2%, 88.5%, 88.5%, and 69.2% of ChAdOx1 vaccinated individuals could neutralize SARS-CoV-2 WT strain, Alpha, Alpha-E484K, Beta, and Delta virus variants, respectively (Fig 2 , A). NT50 values were calculated from neutralization curves by 4-parameter nonlinear regression; a median neutralization curve of each virus is shown in Fig 2, B. Analyses of individual NT50 values demonstrated that the median neutralization capacity induced by the ChAdOx1 vaccine is significantly lower against the Alpha-E484K, Beta, and Delta variants (Alpha-E484K: 107.3; 95% CI, 53.1-224.1; P < .0001; Beta: 179.6; 95% CI, 137.7-224.0; P = .0004; Delta: 64.7; 95% CI, 34.0-200.8; P < .0001) compared to the SARS-CoV-2 WT strain (322.7; 95% CI, 260.6-606.4) (Fig 2, C; see Table E6 in the Online Repository at www.jacionline.org). However, no significantly higher median neutralization titer was detected against the Alpha virus variant (460.9; 95% CI, 291.8-640.0) (Fig 2, C; Table E6). Calculating relative neutralization titers of each SARS-CoV-2 variant against the SARS-CoV-2 WT strain revealed a 70%, 47%, and 82% decrease of neutralizing capacity against Alpha-E484K, Beta, and Delta virus variants, respectively (Fig 2, D, orange). In fact, median NT50 values were only elevated (13%) against the Alpha variant in ChAdOx1 vaccinated individuals (Fig 2, D, blue). In addition, NT90 values were also calculated; they confirmed that among the circulating variants, only Alpha showed elevated neutralization titers compared to the SARS-CoV-2 WT strain (see Fig E2, A-C, and Table E6 in the Online Repository).
Fig 2.
SARS-CoV-2 NT50 neutralization titer analysis using serum samples from fully vaccinated ChAdOx1 (n = 25) participants against SARS-CoV-2 WT, Alpha, Alpha-E484K, Beta, and Delta variants. A, Percentages of vaccinees who tested positive, borderline, or negative for half-maximum neutralization against SARS-CoV-2 WT and variants are shown. B, Neutralization curves of ChAdOx1 immunized individuals including highlighted median neutralization curves for WT and each variant. C, Individual NT50 serum dilutions for all tested SARS-CoV-2 viruses. Statistical significance between WT and variants was determined by Mann-Whitney U test for nonparametric distribution (∗∗∗∗P < .0001). Medians are visualized as red lines together with the interquartile range as error bars. The cutoff value is illustrated by the gray dashed line.D, Determination of NT50 fold changes of SARS-CoV-2 variants relative to WT.
Fig E2.
SARS-CoV-2 neutralization titer (NT90) analysis with serum samples from fully vaccinated ChAdOx1 participants (n = 25) against SARS-CoV-2 WT, Alpha, Alpha-E484K, Beta, and Delta variants. A, Percentages of vaccinees who tested positive (NT90 ≥ 1:16), borderline (1:16 > NT90 ≥ 1:8), or negative (NT90 < 1:8) for NT90 neutralization against SARS-CoV-2 WT and variants. B, Individual NT90 serum dilutions against SARS-CoV-2 WT and variants. Statistical significance between WT and variants was determined by Mann-Whitney U test for nonparametric distribution (∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001). Medians are visualized as red lines together with the interquartile range as error bars. The cutoff value is illustrated by a gray dashed line.C, Determination of NT90 fold changes of SARS-CoV-2 variants relative to WT.
mRNA-based vaccines demonstrate strong neutralization against circulating variants
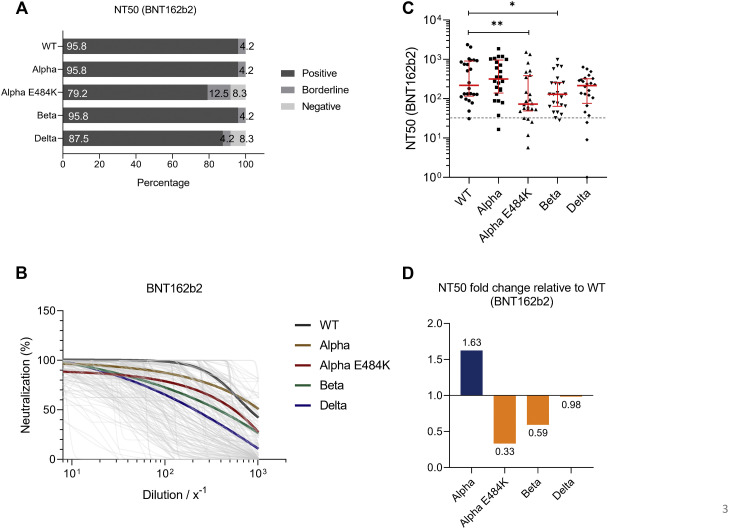
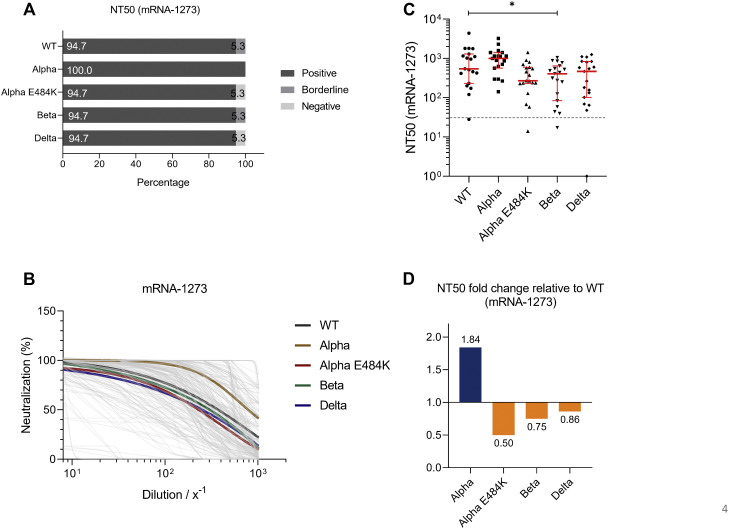
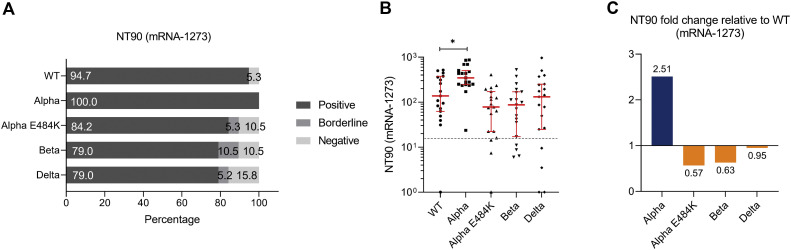
For mRNA-based vaccine BNT162b2, we found that 95.8%, 95.8%, 79.2%, 95.8%, and 87.5% of study participants tested positive for neutralization against the SARS-CoV-2 WT strain or the Alpha, Alpha-E484K, Beta, or Delta virus variants (Fig 3 , A). These already high numbers were even elevated in the mRNA-1273 group because in this group, positive neutralization was determined in 94.7% against the SARS-CoV-2 WT strain, 100.0% against the Alpha variant, 94.7% against the Alpha-E484K variant, 94.7% against the Beta variant, and 94.7% against the Delta variant (Fig 4 , A). NT50 values of individuals vaccinated with BNT162b2 (Fig 3, B) or mRNA-1273 (Fig 4, B) were calculated from neutralization curves by 4-parameter nonlinear regression, and median neutralization curve of each virus is depicted. Comparison of individual NT50 values demonstrated that median neutralization capacity in the BNT162b2 vaccinated group was significantly lower against the Alpha-E484K and Beta variants (Alpha-E484K: 72.8; 95% CI, 53.2-348.8; P = .0053; Beta: 129.2; 95% CI, 64.6-251.0; P = .0303) compared to SARS-CoV-2 WT (217.5; 95% CI, 128.0-854.1) (Fig 3, C; Table E6). No significant difference in median NT50 values was observed between the Delta variant (214.2; 95% CI, 105.1-300.1) and the WT strain (Fig 3, C; Table E6). Interestingly, only NT50 values between the Beta variant (405.7; 95% CI, 83.4-642.9) and the SARS-CoV-2 WT strain (541.9; 95% CI, 232.1-1291.0) were significantly lower (P = .0456), whereas no significant difference between the WT strain and the circulating virus variants (Alpha-E484K: 270.4; 95% CI, 228.3-596.1; Delta: 466.8; 95% CI, 102.1-835.4) were detected for individuals vaccinated with mRNA-1273 (Fig 4, C; Table E6). However, median NT50 values against the Alpha variant for both mRNA-based vaccine groups (BNT162b2: 315.2; 95% CI, 164.5-838.2; mRNA-1273: 996.5; 95% CI, 564.6-1407.0) were slightly increased compared to median NT50 values against WT virus variants (Fig 3, C, and Fig 4, C; Table E6). Analyses of relative neutralization titers of each SARS-CoV-2 variant against the SARS-CoV-2 WT strain in the BNT162b2 group had a 67%, 41%, and 2% decrease of neutralizing capacity against Alpha-E484K, Beta, and Delta virus variants, respectively (Fig 3, D, orange). Calculation of the relative virus neutralization of the mRNA-1273 group showed a 50%, 25%, and 14% reduced neutralization against Alpha-E484K, Beta, and Delta virus variants, respectively (Fig 4, D, orange). Individuals vaccinated with both mRNA vaccines acquired elevated neutralization capacity against SARS-CoV-2 Alpha variant up to 82% compared to the SARS-CoV-2 WT strain (Fig 3, D, and Fig 4, D, blue). In addition, NT90 values were calculated for the BNT162b2 and mRNA-1273 groups (see Fig E3, Fig E4, and Table E6, right, in the Online Repository at www.jacionline.org). These data confirmed that for the mRNA-1273 group, elevated neutralization titers compared to the SARS-CoV-2 WT strain were only detected against the Alpha variant (Fig E4, A-C; Table E6). In contrast, the NT90 values of the BNT162b2 group showed a reduction of relative neutralization capacity for the SARS-CoV-2 Alpha variant (Fig E3, A-C; Table E6).
Fig 3.
SARS-CoV-2 NT50 analysis using serum samples from fully vaccinated BNT162b2 (n = 24) individuals against SARS-CoV-2 WT, Alpha, Alpha-E484K, Beta, and Delta variants. A, Percentages of vaccinees who tested positive, borderline, or negative for half-maximum neutralization against SARS-CoV-2 WT and variants. B, Neutralization curves of BNT162b2 immunized participants including highlighted median neutralization curves for WT and each variant. C, Individual NT50 serum dilutions for all tested SARS-CoV-2 viruses. Statistical significance between WT and variants was determined by Mann-Whitney U test for nonparametric distribution (∗P < .05; ∗∗P < .01). Medians are visualized as red lines together with the interquartile range as error bars. The cutoff value is illustrated by a gray dashed line.D, Determination of NT50 fold changes of SARS-CoV-2 variants relative to WT.
Fig 4.
SARS-CoV-2 NT50 analysis using serum samples from fully vaccinated mRNA-1273 (n = 23) individuals against SARS-CoV-2 WT, Alpha, Alpha-E484K, Beta, and Delta variants. A, Percentages of vaccinees who tested positive, borderline, or negative for half-maximum neutralization against SARS-CoV-2 WT and variants. B, Neutralization curves of mRNA-1273 immunized participants including highlighted median neutralization curves for WT and each variant. C, Individual NT50 serum dilutions for all tested SARS-CoV-2 viruses. Statistical significance between WT and variants was determined by Mann-Whitney U test for nonparametric distribution (∗P < .05; ∗∗P < .01). Medians are visualized as red lines together with the interquartile range as error bars. The cutoff value is illustrated by a gray dashed line.D, Determination of NT50 fold changes of SARS-CoV-2 variants relative to WT.
Fig E3.
SARS-CoV-2 neutralization titer (NT90) analysis with serum samples from fully immunized BNT162b2 participants (n = 24) against SARS-CoV-2 WT, Alpha, Alpha-E484K, Beta, and Delta variants. A, Percentages of vaccinees who tested positive (NT90 ≥ 1:16), borderline (1:16 > NT90 ≥ 1:8), or negative (NT90 < 1:8) for NT90 neutralization against SARS-CoV-2 WT and variants. B, Individual NT90 serum dilutions against SARS-CoV-2 WT and variants. Statistical significance between WT and variants was determined by Mann-Whitney U test for nonparametric distribution (∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001). Medians are visualized as red lines together with the interquartile range as error bars. The cutoff value is illustrated by a gray dashed line.C, Determination of NT90 fold changes of SARS-CoV-2 variants relative to WT.
Fig E4.
SARS-CoV-2 neutralization titer (NT90) analysis with serum samples from fully vaccinated mRNA-1273 participants (n = 23) against SARS-CoV-2 WT, Alpha, Alpha-E484K, Beta, and Delta variants. A, Percentages of vaccinees tested positive (NT90 ≥ 1:16), borderline (1:16 > NT90 ≥ 1:8), or negative (NT90 < 1:8) for NT90 neutralization against SARS-CoV-2 WT and variants. B, Individual NT90 serum dilutions against SARS-CoV-2 WT and variants. Statistical significance between WT and variants was determined by Mann-Whitney U test for nonparametric distribution (∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001). Medians are visualized as red lines together with the interquartile range as error bars. The cutoff value is illustrated by a gray dashed line.C, Determination of NT90 fold changes of SARS-CoV-2 variants relative to WT.
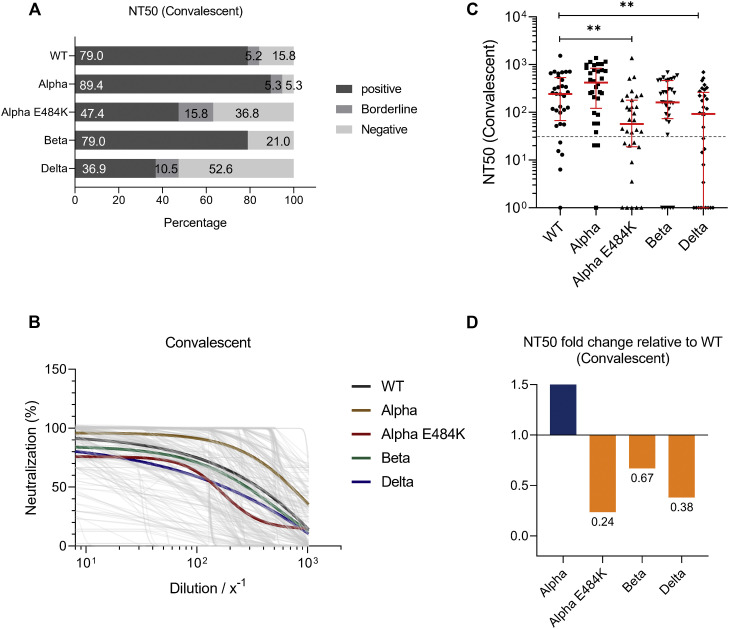
Neutralization capacity of COVID-19 convalescent patients
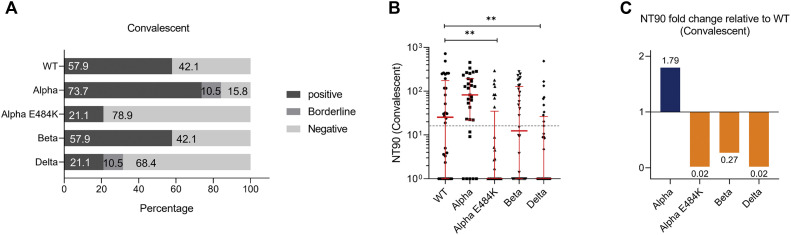
Serum samples from recovered and proven COVID-19 patients were analyzed, and NT50 values were analyzed. In this patient group, we found that 79.0%, 89.4%, 47.4%, 79.0%, and 36.9% of samples from convalescent individuals could neutralize the SARS-CoV-2 WT strain or Alpha, Alpha-E484K, Beta, or Delta virus variants, respectively (Fig 5 , A). NT50 values were calculated from neutralization curves by 4-parameter nonlinear regression and a median neutralization curve of each SARS-CoV-2 WT; a circulating variant is depicted in Fig 5, B. Individual NT50 values within the convalescent group showed that the neutralization capacity against the SARS-CoV-2 Alpha variant (420.0; 95% CI, 208.3-769.8.0) was higher compared to the WT strain (241.9; 95% CI, 109.1-353.8) (Fig 5, C; Table E6). Furthermore, median NT50 values were lower for the Beta variant (161.7; 95% CI, 92.4-308.5) and significantly decreased in convalescent samples against the Alpha-E484K and Delta variants (Alpha-E484K: 56.9; 95% CI, 22.4-129.8; P = .0055; Delta: 92.0; 95% CI, 3.4-221.3; P = .0013) (Fig 5, C; Table E6). In this group, the highest median NT50 values were detected against the SARS-CoV-2 Alpha variant, while the median neutralization titers for the Alpha-E484K and Delta variants were the lowest (Fig 5, C; Table E6). Calculating relative neutralization titers of each SARS-CoV-2 variant against the SARS-CoV-2 WT strain revealed a 93%, 16%, and 95% decrease of neutralizing capacity against the Alpha-E484K, Beta, and Delta virus variants, respectively (Fig 5, D, orange). In comparison, median NT50 values were elevated by 25% against the Alpha variant (Fig 5, D, blue). Analyses of NT90 values confirmed that among the circulating variants, only the Alpha variant resulted in elevated neutralization titers compared to the SARS-CoV-2 WT strain (see Fig E5, A-C, and Table E6 in the Online Repository at www.jacionline.org).
Fig 5.
SARS-CoV-2 NT50 analysis using sera from convalescent patients (n = 24) against SARS-CoV-2 WT, Alpha, Alpha-E484K, Beta, and Delta variants. A, Percentages of COVID-19 patients who tested positive, borderline, or negative for half-maximum neutralization against SARS-CoV-2 WT and variants. B, Neutralization curves of patients including highlighted median neutralization curves for WT and each variant. C, Individual NT50 serum dilutions for all tested SARS-CoV-2 viruses. Statistical significance between WT and variants was determined by Mann-Whitney U test for nonparametric distribution (∗P < .05; ∗∗P < .01). Medians are visualized as red lines together with the interquartile range as error bars. The cutoff value is illustrated by a gray dashed line.D, Determination of NT50 fold changes of SARS-CoV-2 variants relative to WT.
Fig E5.
SARS-CoV-2 neutralization titer (NT90) analysis with serum samples from recovered COVID-19 patients (n = 24) against SARS-CoV-2 WT, Alpha, Alpha-E484K, Beta, and Delta variants. A, Percentages of vaccinees who tested positive (NT90 ≥ 1:16), borderline (1:16 > NT90 ≥ 1:8), or negative (NT90 < 1:8) for NT90 neutralization against SARS-CoV-2 WT and variants. B, Individual NT90 serum dilutions against SARS-CoV-2 WT and variants. Statistical significance between WT and variants was determined by Mann-Whitney U test for nonparametric distribution (∗P < .05). Medians are visualized as red lines together with the interquartile range as error bars. The cutoff value is illustrated by a gray dashed line.C, Determination of NT90 fold changes of SARS-CoV-2 variants relative to WT.
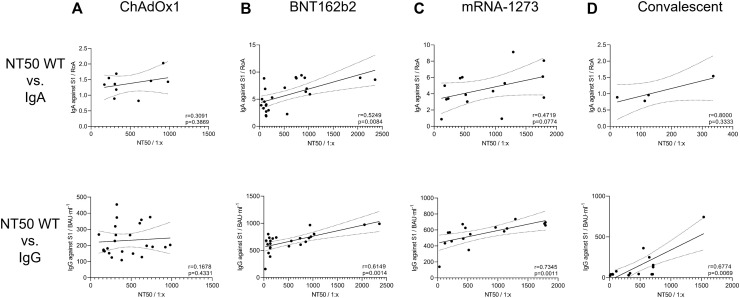
Positive correlation between IgA and IgG antibody titers and NT50 values against SARS-CoV-2 WT virus
Results from antibody titers and neutralization assays (NT50 values) observed in COVID-19 vaccinated or convalescents individuals were statistically analyzed to identify a correlation between higher amounts of IgA or IgG antibodies and enhanced neutralization of SARS-CoV-2 WT strain. Therefore, we next performed correlation analyzes between antibody titers and corresponding NT50 values from each study participant by 2-tailed nonparametric Spearman correlation. For ChAdOx1 vaccinated individuals, we found a nonsignificant IgA/NT50 and IgG/NT50 correlation of r = 0.3091 (P = .3869) and r = 0.1678 (P = .4331), respectively (Fig 6 , A). Furthermore, a significant, positive relation for BNT162b2 (r = 0.5249; P = .0084) and a positive correlation for mRNA-1273 (r = 0.4719; P = .0774) immunized, but not for convalescent (r = 0.8; P = .333), individuals (Fig 6, B-D, top) were found for IgA/NT50 correlation. Similar results were obtained for calculation of IgG/NT50 correlations. Here, we found a significant, positive relation for BNT162b2 immunized (r = 0.6149; P = .0014), mRNA-1273 immunized (r = 0.73459; P = .0011), and also convalescent (r = 0.67749; P = .0069) individuals (Fig 6, B-D, bottom). The correlation between total SARS-CoV-2-RBD–specific Ig and NT50 values was not calculated for vaccinated individuals because all antibody titers were detected in the saturated range of the ELISA. However, a significant, positive relation between total RBD-specific antibodies and NT50 values was found for convalescent patients (data not shown).
Fig 6.
Correlation between SARS-CoV-2–specific antibody titers and corresponding NT50 values against SARS-CoV-2 WT. Dependence between SARS-CoV-2–specific IgA (top) and IgG (bottom) and NT50 values from SARS-CoV-2 WT virus was calculated for (A) ChAdOx1, (B) BNT162b2, and (C) mRNA-1273 vaccinated and (D) convalescent participants by nonparametric Spearman correlation analyses. Correlation of Ig/NT50. To improve visualization of the trend, a linear regression with 95% confidence interval (CI) was plotted. IgA titers are presented as RoA; IgG titers, binding antibody unit per milliliter of serum (BAU∙mL−1); and NT50, dilution factor for serum (1:x).
Comparison of NT50 values of COVID-19 vaccinated individuals and convalescent patients
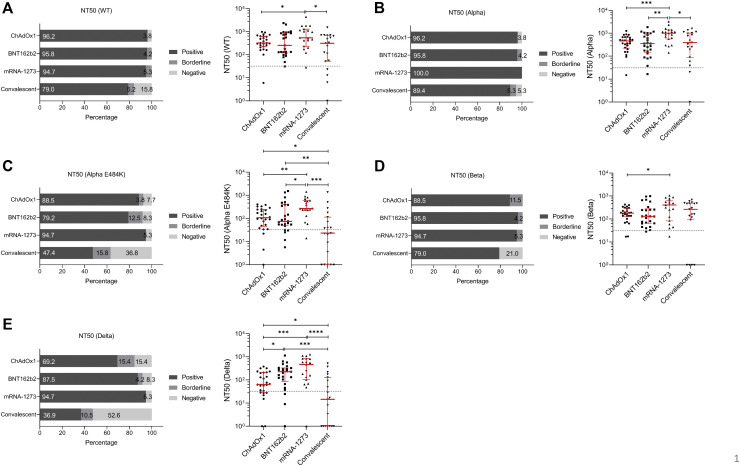
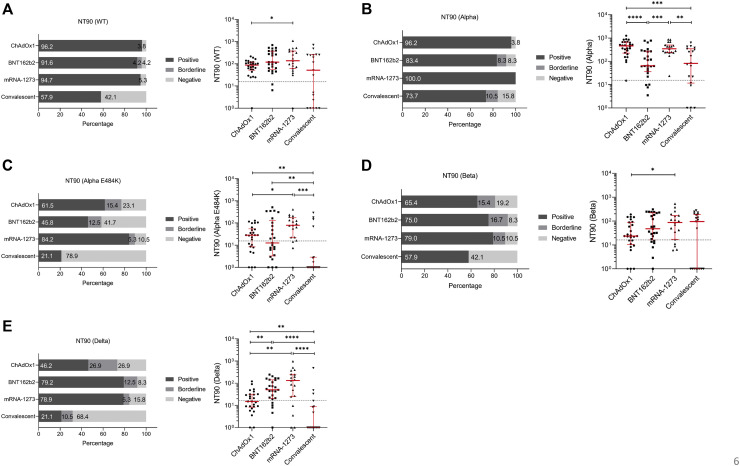
Finally, we compared the efficacy of SARS-CoV-2 neutralization by sera from individuals immunized with mRNA- or vector-based vaccines and convalescent patients for SARS-CoV-2 and circulating variants. For the SARS-CoV-2 WT strain, we found that 79.0% of recovered patients had sufficient neutralization, while more than 94% of vaccinated individuals could neutralize the WT strain (Fig 7 , A, left). Analyses of the individual data revealed significant differences between convalescent patients and mRNA-1273 vaccinees (r = 0.0228) as well as between ChAdOx1 and mRNA-1273 vaccinees (r = 0.0454) (Fig 7, A, right). Individuals vaccinated with BNT162b2 showed similar NT50 results to the COVID-19 recovered group (Fig 7, A, right). Ample neutralization against the SARS-CoV-2 Alpha variant was demonstrated in convalescent patients (89.4%) as well as in BNT162b2 (95.8%) or ChAdOx1 (96.2%) immunized individuals (Fig 7, B, left). All mRNA-1273 vaccinated study participants were able to neutralize the SARS-CoV-2 Alpha variant (Fig 7, B, left); analyses of the individual data showed significantly higher neutralization titers for mRNA-1273 compared to convalescent patients (P = .0248) or ChAdOx1 (P = .0009) or BNT162b2 (P = .0038) vaccinees (Fig 7, B, right). Neutralization assays performed using the Beta virus variant demonstrated that 79.0% of recovered and 88.5% of ChAdOx1 vaccinated individuals tested positive, while more than 94% of participants immunized with mRNA-based vaccines could efficiently neutralize the virus (Fig 7, D, left). No significant differences were detected between the BNT162b2, mRNA-1273, or convalescent groups, while the mRNA-1273 vaccinated group had significantly higher NT50 values compared to the ChAdOx1 group (P = .0388) (Fig 7, D, right). For the SARS-CoV-2 Alpha-E484K variant, NT50 values for recovered patients or patients vaccinated with mRNA-1273, BNT162b2, and ChAdOx1 were 47.4%, 94.7, 79.2%, and 88.5%, respectively (Fig 7, C, left). Further analyses revealed that mRNA-1273 demonstrated significantly higher neutralization titers compared to ChAdOx1 (P = .0015) or BNT162b2 (P = .0194) immunized or recovered participants (P = .0001) (Fig 7, C, right). Significantly lower NT50 values were also found between convalescent patients and the ChAdOx1 (P = .0162) and the BNT162b2 (P = .0080) groups (Fig 7, C, right). This already impaired neutralization capacity against the Alpha-E484K variant is even reduced when examining the Delta virus variant. Indeed, only 50.0% of convalescent and 69.2% of ChAdOx1 vaccinated individuals could efficiently neutralize this SARS-CoV-2 variant (Fig 7, E, left). Best results were experienced by those vaccinated with mRNA-1273 (94.7%), followed by BNT162b2 (87.5%) (Fig 7, E, left). In addition, median NT50 values were significantly higher in mRNA-1273 immunized people compared to ChAdOx1 vaccinated (P = .0009) or convalescent (P < .0001) individuals (Fig 7, E, right). Furthermore, the BNT162b2 group also showed a significantly higher median NT50 value than ChAdOx1 vaccinees (P = .0130) and those who recovered from COVID-19 (P = .0005) (Fig 7, E, right). Significantly lower NT50 values were found between convalescent patients and the ChAdOx1 group (P = .0282) (Fig 7, E, right). Analyses of NT90 values confirmed that among the tested groups, individuals immunized with the mRNA-based vaccines showed elevated neutralization titers compared to ChAdOx1 vaccinated or recovered participants (see Fig E6 in the Online Repository at www.jacionline.org).
Fig 7.
Comparison of neutralization titers (NT50 values) from individuals fully vaccinated with ChAdOx1, BNT162b2, or mRNA-1273 and convalescent patients. Percentages (left) and individual NT50 serum dilutions (right) of vaccinated or convalescent participants who tested positive, borderline, and negative against (A) WT, (B) Alpha, (C) Alpha-E484K, (D) Beta, and (E) Delta variants. Statistical significance between the 4 cohorts was determined by Mann-Whitney U test for nonparametric distribution (∗P < .05; ∗∗P < .01; ∗∗∗P < .001). Medians are visualized as red lines together with the interquartile range as error bar. Cutoff values are illustrated by a gray dashed line.
Fig E6.
Comparison of neutralization titers (NT90 values) from individuals fully vaccinated with ChAdOx1, BNT162b2, or mRNA-1273 and convalescent patients. Percentages (left) and individual NT90 values (right) of vaccinated or convalescent participants who tested positive (NT90 ≥ 1:16), borderline (1:16 > NT90 ≥ 1:8), and negative (NT90 < 1:8) for 90% neutralization positive, borderline, and negative against (A) WT, (B) Alpha, (C) Alpha-E484K, (D) Beta, and (E) Delta variants. Statistical significance between the 4 cohorts was determined by Mann-Whitney U test for nonparametric distribution (∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001). Medians are visualized as red lines together with the interquartile range as error bar. Cutoff values are illustrated by a gray dashed line.
Discussion
In this study, we compared humoral immune responses and neutralization titers in individuals immunized with 3 different COVID-19 vaccines and in convalescent patients. Most of the studies on humoral immune responses in COVID-19 vaccinated or convalescent patients investigated serum IgG levels, but there is compelling evidence that secretory and circulating IgA play an important role in immunity to COVID-19.22 For that reason, we here investigated IgG and IgA levels in serum samples of vaccinated and convalescent individuals. We observed a robust IgG response to viral spike protein and its RBD in all vaccinated participants; however, the highest median antibody titers were detected in both of the available mRNA-based vaccines. This is in accordance with other published data, which showed that 2 vaccine doses resulted in high antibody titers across all ages with receipt of any of ChAdOx1, BNT162b2, or mRNA-1273.23 , 24 We also found that antibody levels in convalescent patients have a high titer variation, which has been previously reported and which correlated with the duration and severity of clinical symptoms, but not with patient age.25
In case of SARS-CoV-2–specific serum IgA responses, we could confirm the highest titers in mRNA-based vaccines compared to vector-based vaccine or convalescent patients. IgA antibodies are a heterogeneous group of immunoglobulins and occur in secretory, polymeric, and monomeric forms.26 Although studies have demonstrated that there is only a weak correlation between mucosal IgA and serum IgA antibodies because they are mostly generated locally in the salivary glands, some studies have noted that mucosal IgAs are even detectable in seronegative IgA convalescent patients.27 Therefore, the IgA levels that we determined here in vaccinated people might not reflect the situation in the mucosa. In fact, it is possible that the number of IgA-positive individuals was indeed higher. Sterlin et al28 found that specific IgA concentrations in the serum decreased notably 1 month after onset of symptoms, whereas IgA levels remained detectable in saliva longer. Furthermore, anti–SARS-CoV-2 RBD IgA were also detected in mRNA-based vaccine recipients, and salivary IgAs in both groups displayed detectable neutralizing abilities.29 , 30 Whether a strong vaccine-induced mucosal immunity conducted by IgG and IgA levels in mucus and saliva may contribute to protection of infection and reduced person-to-person transmission of SARS-CoV-2 remains to be investigated. A recent study investigating the relationship of selective IgA deficiency and severe COVID-19 infection found a 7.7-fold higher risk of severe COVID-19 in patients with this deficiency.22 Our data revealed the existence of a significant, positive relation between Ig (IgA and IgG) and NT50 values for immunized and convalescent individuals, suggesting that the observed humoral responses were protective. A correlation between neutralization titers after COVID-19 or SARS-CoV-2 vaccination and protection against SARS-CoV-2 infection has been demonstrated by several studies.31 , 32 Accordingly, the strength of the humoral response after illness or vaccination might be clinically relevant.24 Of note, all of these studies used the SARS-CoV-2 WT virus or pseudotyped viruses for determining neutralization abilities, but novel SARS-CoV-2 variants with the potential of higher transmission and more severe infection have emerged.21 , 33 , 34 As a result of mutations in the RBD region of the viral spike protein, these VOCs or variants of interest have the potential to escape antibody binding and neutralization efficacy, which we analyzed here using sera from vaccinated and convalescent individuals. We found that all 3 investigated vaccines could neutralize the Alpha, Alpha-E484K, Beta, and Delta variants in addition to the SARS-CoV-2 WT virus, but in some individuals, only low or no neutralization was detected. In our cohort, neutralization against the Alpha-E484K and Delta variants was strongly reduced, especially in individuals immunized with the vector-based vaccine ChAdOx1.
Other studies support our findings for the Delta variant and have observed an efficacy of 88% for BNT162b2 and 67% for ChAdOx1.35 Interestingly, we found an increased effectiveness for all vaccines and convalescent patients for the Alpha variant, which contradicts the current literature showing reduced protection for the Alpha variant.16 , 35 Here we also studied the performance of a second mRNA-based vaccine and found that mRNA-1273 had the highest neutralization compared to BNT162b2 and ChAdOx1 vaccinees or convalescent patients. At the moment, only a few articles on mRNA-1273 are available showing that mRNA vaccines are highly effective, with a slightly higher efficacy for mRNA-1273.36 , 37 In addition, Steensels et al24 observed higher total Ig titers against RBD of SARS-CoV-2 in participants vaccinated with 2 doses of mRNA-1273 compared to those vaccinated with BNT162b2. A possible explanation for this might be the higher mRNA content in mRNA-1273 compared to BNT162b2, and the longer interval between priming and boosting for mRNA-12733 (4 weeks, vs 3 weeks for BNT162b2). In comparison, here we did not find differences of total Igs against RBD between mRNA-1273 and BNT162b2, and we detected higher IgG and IgA for BNT162b2.
Limitations of this study include the small number of study participants and the lack of data on cellular immunity, which we previously investigated in COVID-19 patients with mild, severe, and critical disease progression.38 The effect of cellular immunity in protection against infection and prevention of virus transmission has been studied and remains a subject of ongoing investigation.39 , 40 This is important because T cells might limit disease progression in case of low neutralizing antibody levels, and generation of T-cell responses differs between mRNA- and vector-based vaccines.41 , 42 Also, cellular immunity in COVID-19 convalescent patients has to be determined in more detail because our data also showed that antibody titers and neutralization ability ranged widely between this group’s individuals. As a result of these heterogeneous results within the COVID-19 convalescent group, it is still difficult to assess how and if protection of recovered individuals is facilitated. Novel COVID-19 vaccine formulations using various platforms and new vaccine technologies are currently being developed to deal with the ongoing pandemic’s new challenges.43 New formulations might include a nasal vaccine application in an attempt to enhance innate and adaptive mucosal immunity.44 Identifying whether novel vaccine candidates can also increase efficacy against emerging SARS-CoV-2 virus variants and reduce breakthrough infections is a priority for future investigations.
Key messages.
-
•
The mRNA-1273 vaccine showed the highest neutralization compared to BNT162b2 and ChAdOx1.
-
•
COVID-19 convalescent patients demonstrated a lower range of antibody or neutralization titers.
-
•
Significant, positive relations were found between antibodies and NT50 values.
Acknowledgments
We thank technicians Isolde Enz, Mariam Ghorbani, Ruth Mader, Bettina Sartori, and Tanja Triendl (University Hospital of Internal Medicine II, Medical University of Innsbruck, Innsbruck, Austria), as well as Gabriel Diem (Institute of Hygiene and Medical Microbiology, Medical University of Innsbruck) for their valuable help and support regarding this study.
Footnotes
The first 2 authors contributed equally to this article, and both should be considered first author.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA (NIBSC 19/304) was obtained from the National Institute for Biological Standards and Control, United Kingdom. The following reagents were deposited by the US Centers for Disease Control and Prevention and obtained through BEI Resources, National Institute of Allergy and Infectious Diseases, US National Institutes of Health: SARS-related coronavirus 2, isolate USA-WA1/2020 NR-52281; SARS-related coronavirus 2, isolate hCoV-19/England/204820464/2020, NR-54000, contributed by Bassam Hallis; and SARS-related coronavirus 2, isolate hCoV-19/South Africa/KRISP-K005325/2020, NR-54009, contributed by Alex Sigal and Tulio de Oliveira. The authors were supported by the Austrian Science Fund (FWF; P34070-B to W.P. and P33510-B to D.W.), the Anniversary Fund of the Austrian National Bank (OeNB; P17614 to W.P., P17633 to D.W.), and the state of Tyrol (70454 to W.P.).
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Appendix
Table E1.
Characteristics from 33 individuals fully vaccinated with ChAdOx1
| Patient ID | Age (years) | Sex | Days after second dose | NT analysis | Total anti-RBD Ig (Wantai) |
Anti-S1 IgG (Euroimmun) |
Anti-S1 IgA (Euroimmun) |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| Results | RoA | Results | Concentration (BAU∙mL−1) | Results | RoA | |||||
| A1 | 25 | F | 29 | Yes | Positive | 17.64 | Positive | 337.88 | Positive | 0.82 |
| A2 | 26 | M | 29 | Yes | Positive | 17.89 | Positive | 127.84 | Negative | 0.55 |
| A3 | 28 | M | 29 | Yes | Positive | 17.82 | Positive | 203.20 | Positive | 1.43 |
| A4 | 28 | M | 29 | Yes | Positive | 17.58 | Positive | 264.62 | Negative | 0.49 |
| A5 | 28 | F | 29 | Yes | Positive | 17.86 | Positive | 264.39 | Negative | 0.50 |
| A6 | 29 | M | 29 | Yes | Positive | 18.15 | Positive | 162.23 | Negative | 0.49 |
| A7 | 30 | F | 29 | Yes | Positive | 17.91 | Negative | 22.90 | Negative | 0.28 |
| A8 | 35 | M | 29 | Yes | Positive | 18.96 | Positive | 181.26 | Negative | 0.61 |
| A9 | 38 | F | 29 | Yes | Positive | 17.66 | Positive | 189.57 | Positive | 2.03 |
| A10 | 39 | M | 29 | Yes | Positive | 17.90 | Positive | 227.50 | Positive | 0.89 |
| A11 | 39 | M | 29 | Yes | Positive | 18.36 | Positive | 191.57 | Positive | 1.46 |
| A12 | 41 | F | 29 | Yes | Positive | 18.35 | Positive | 173.14 | Negative | 0.37 |
| A13 | 42 | M | 29 | Yes | Positive | 18.73 | Positive | 164.09 | Positive | 1.34 |
| A14 | 42 | M | 29 | Yes | Positive | 19.13 | Positive | 152.04 | Positive | 1.55 |
| A15 | 45 | F | 29 | Yes | Positive | 20.24 | Positive | 373.22 | Positive | 1.69 |
| A16 | 47 | M | 29 | Yes | Positive | 19.28 | Positive | 125.39 | Negative | 0.36 |
| A17 | 47 | F | 29 | Yes | Positive | 17.85 | Positive | 168.21 | Negative | 0.60 |
| A18 | 47 | F | 29 | Yes | Positive | 18.67 | Positive | 453.82 | Positive | 1.18 |
| A19 | 50 | F | 29 | Yes | Positive | 18.18 | Positive | 375.76 | Negative | 0.55 |
| A20 | 56 | F | 29 | Yes | Positive | 18.45 | Positive | 267.71 | Positive | 4.44 |
| A21 | 57 | F | 29 | Yes | Positive | 19.20 | Positive | 318.41 | Positive | 1.36 |
| A22 | 58 | F | 29 | Yes | Positive | 18.64 | Positive | 197.86 | Negative | 0.51 |
| A23 | 58 | F | 36 | Yes | Positive | 17.55 | Positive | 149.36 | Negative | 0.26 |
| A24 | 61 | M | 36 | Yes | Positive | 17.92 | Positive | 358.77 | Negative | 0.63 |
| A25 | 62 | F | 29 | Yes | Positive | 18.86 | Positive | 108.98 | NA | NA |
| A26 | 51 | M | 29 | No | Positive | 18.58 | Positive | 540.93 | Positive | 6.11 |
| A27 | 42 | M | 29 | No | Positive | 18.81 | Positive | 259.92 | Positive | 1.16 |
| A28 | 56 | M | 29 | No | Positive | 18.07 | Positive | 429.08 | Positive | 0.82 |
| A29 | 32 | F | 29 | No | Positive | 18.36 | Positive | 534.19 | Positive | 2.36 |
| A30 | 47 | F | 29 | No | Positive | 17.86 | Positive | 423.68 | Negative | 0.67 |
| A31 | 51 | F | 34 | No | Positive | 19.79 | Positive | 259.45 | Negative | 0.51 |
| A32 | 39 | F | 33 | No | Positive | 19.21 | Positive | 462.21 | Positive | 2.36 |
| A33 | 51 | F | 33 | No | Positive | 18.27 | Positive | 350.28 | Negative | 0.61 |
Results for total Ig against SARS-CoV-2 RBD and for IgA against SARS-CoV-2 domain S1 are shown as RoA. For IgG against SARS-CoV-2 domain, S1 results are presented in binding antibody units per milliliter of serum (BAU∙mL−1). Serum samples used for the neutralization experiments are indicated in “NT analysis” column. The average age of the participants was 43 years; the percentage of women was 58%. All samples from ChAdOx1 vaccinees were taken between 29 and 36 days after receipt of the second dose. NA, Not applicable.
Table E2.
Characteristics from 31 individuals fully vaccinated with BNT162b2
| Patient ID | Age (years) | Sex | Days after second dose | NT analysis | Total anti-RBD Ig (Wantai) |
Anti-S1 IgG (Euroimmun) |
Anti-S1 IgA (Euroimmun) |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| Results | RoA | Results | Concentration (BAU∙mL−1) | Results | RoA | |||||
| A1 | 25 | F | 29 | Yes | Positive | 17.64 | Positive | 337.88 | Positive | 0.82 |
| A2 | 26 | M | 29 | Yes | Positive | 17.89 | Positive | 127.84 | Negative | 0.55 |
| A3 | 28 | M | 29 | Yes | Positive | 17.82 | Positive | 203.20 | Positive | 1.43 |
| A4 | 28 | M | 29 | Yes | Positive | 17.58 | Positive | 264.62 | Negative | 0.49 |
| A5 | 28 | F | 29 | Yes | Positive | 17.86 | Positive | 264.39 | Negative | 0.50 |
| A6 | 29 | M | 29 | Yes | Positive | 18.15 | Positive | 162.23 | Negative | 0.49 |
| A7 | 30 | F | 29 | Yes | Positive | 17.91 | Negative | 22.90 | Negative | 0.28 |
| A8 | 35 | M | 29 | Yes | Positive | 18.96 | Positive | 181.26 | Negative | 0.61 |
| A9 | 38 | F | 29 | Yes | Positive | 17.66 | Positive | 189.57 | Positive | 2.03 |
| A10 | 39 | M | 29 | Yes | Positive | 17.90 | Positive | 227.50 | Positive | 0.89 |
| A11 | 39 | M | 29 | Yes | Positive | 18.36 | Positive | 191.57 | Positive | 1.46 |
| A12 | 41 | F | 29 | Yes | Positive | 18.35 | Positive | 173.14 | Negative | 0.37 |
| A13 | 42 | M | 29 | Yes | Positive | 18.73 | Positive | 164.09 | Positive | 1.34 |
| A14 | 42 | M | 29 | Yes | Positive | 19.13 | Positive | 152.04 | Positive | 1.55 |
| A15 | 45 | F | 29 | Yes | Positive | 20.24 | Positive | 373.22 | Positive | 1.69 |
| A16 | 47 | M | 29 | Yes | Positive | 19.28 | Positive | 125.39 | Negative | 0.36 |
| A17 | 47 | F | 29 | Yes | Positive | 17.85 | Positive | 168.21 | Negative | 0.60 |
| A18 | 47 | F | 29 | Yes | Positive | 18.67 | Positive | 453.82 | Positive | 1.18 |
| A19 | 50 | F | 29 | Yes | Positive | 18.18 | Positive | 375.76 | Negative | 0.55 |
| A20 | 56 | F | 29 | Yes | Positive | 18.45 | Positive | 267.71 | Positive | 4.44 |
| A21 | 57 | F | 29 | Yes | Positive | 19.20 | Positive | 318.41 | Positive | 1.36 |
| A22 | 58 | F | 29 | Yes | Positive | 18.64 | Positive | 197.86 | Negative | 0.51 |
| A23 | 58 | F | 36 | Yes | Positive | 17.55 | Positive | 149.36 | Negative | 0.26 |
| A24 | 61 | M | 36 | Yes | Positive | 17.92 | Positive | 358.77 | Negative | 0.63 |
| A25 | 62 | F | 29 | Yes | Positive | 18.86 | Positive | 108.98 | NA | NA |
| A26 | 51 | M | 29 | No | Positive | 18.58 | Positive | 540.93 | Positive | 6.11 |
| A27 | 42 | M | 29 | No | Positive | 18.81 | Positive | 259.92 | Positive | 1.16 |
| A28 | 56 | M | 29 | No | Positive | 18.07 | Positive | 429.08 | Positive | 0.82 |
| A29 | 32 | F | 29 | No | Positive | 18.36 | Positive | 534.19 | Positive | 2.36 |
| A30 | 47 | F | 29 | No | Positive | 17.86 | Positive | 423.68 | Negative | 0.67 |
| A31 | 51 | F | 34 | No | Positive | 19.79 | Positive | 259.45 | Negative | 0.51 |
| A32 | 39 | F | 33 | No | Positive | 19.21 | Positive | 462.21 | Positive | 2.36 |
| A33 | 51 | F | 33 | No | Positive | 18.27 | Positive | 350.28 | Negative | 0.61 |
Results for total Ig against SARS-CoV-2 RBD and for IgA against SARS-CoV-2 domain S1 are shown as RoA. For IgG against SARS-CoV-2 domain, S1 results are presented in binding antibody units per milliliter of serum (BAU∙mL−1). Serum samples used for the neutralization experiments are indicated in “NT analysis” column. The average age of the participants was 43 years; the percentage of women was 58%. All samples of BNT162b2 vaccinees were taken between 29 and 36 days after the second dose. NA, Not applicable.
Table E3.
Characteristics of 23 individuals fully vaccinated with mRNA-1273
| Patient ID | Age (years) | Sex | Days after second dose | NT analysis | Total anti-RBD Ig (Wantai) |
Anti-S1 IgG (Euroimmun) |
Anti-S1 IgA (Euroimmun) |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| Results | RoA | Results | Concentration (BAU∙mL−1) | Results | RoA | |||||
| M1 | 31 | M | 104 | Yes | Positive | 17.55 | Positive | 626.20 | Positive | 6.03 |
| M2 | 33 | F | 50 | Yes | Positive | 17.65 | Positive | 433.91 | Positive | 0.87 |
| M3 | 47 | F | 50 | Yes | Positive | 17.41 | Positive | 139.59 | Negative | 0.16 |
| M4 | 64 | M | 57 | Yes | Positive | 17.09 | Positive | 492.38 | Positive | 5.91 |
| M5 | 66 | F | 50 | Yes | Positive | 18.75 | Positive | 736.14 | Positive | 9.13 |
| M6 | 36 | M | 50 | Yes | Positive | 18.51 | Positive | 672.50 | Negative | 0.18 |
| M7 | 57 | M | 50 | Yes | Positive | 18.08 | Positive | 569.36 | Positive | 3.31 |
| M8 | 54 | M | 50 | Yes | Positive | 18.27 | Positive | 458.47 | Positive | 3.40 |
| M9 | 43 | F | 50 | Yes | Positive | 18.54 | Positive | 566.93 | Positive | 4.99 |
| M10 | 46 | F | 52 | Yes | Positive | 18.66 | Positive | 658.25 | Positive | 3.53 |
| M11 | 62 | F | 52 | Yes | Positive | 18.08 | Positive | 695.31 | Positive | 8.07 |
| M12 | 29 | M | 52 | Yes | Positive | 20.48 | Positive | 738.90 | Negative | 0.23 |
| M13 | 63 | M | 52 | Yes | Positive | 18.56 | Positive | 547.37 | Positive | 3.03 |
| M14 | 63 | F | 52 | Yes | Positive | 18.53 | Positive | 679.68 | Positive | 6.10 |
| M15 | 54 | F | 57 | Yes | Positive | 18.15 | Positive | 349.20 | Positive | 3.88 |
| M16 | 33 | F | 57 | Yes | Positive | 19.48 | Positive | 632.40 | Positive | 4.32 |
| M17 | 63 | F | 57 | Yes | Positive | 18.13 | Positive | 583.31 | Positive | 0.93 |
| M18 | 50 | M | 57 | Yes | Positive | 18.55 | Positive | 618.59 | Positive | 5.29 |
| M19 | 61 | F | 57 | No | Positive | 19.36 | Positive | 672.88 | Positive | 7.12 |
Results for total Ig against SARS-CoV-2 RBD and for IgA against SARS-CoV-2 domain S1 are shown as RoA. For IgG against SARS-CoV-2 domain, S1 results are presented in binding antibody units per milliliter of serum (BAU∙mL−1). Serum samples used for the neutralization experiments are indicated in “NT analysis” column. The average age of the participants was 51 years; the percentage of women was 58%. All samples of mRNA-1273 vaccinees were taken between 50 and 104 days after the second dose.
Table E4.
Characteristics of 29 SARS-CoV-2 convalescent patients
| Patient ID | Age (years) | Sex | Days after second dose | NT analysis | Total anti-RBD Ig (Wantai) |
Anti-S1 IgG (Euroimmun) |
Anti-S1 IgA (Euroimmun) |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| Results | RoA | Results | Concentration (BAU∙mL−1) | Results | RoA | |||||
| C1 | 22 | F | 107 | Yes | Positive | 16.57 | Positive | 54.15 | Positive | 1.54 |
| C2 | 23 | F | 107 | Yes | Positive | 16.44 | Borderline | 30.29 | Negative | 0.27 |
| C3 | 27 | M | 22 | Yes | Positive | 6.00 | Positive | 77.58 | Borderline | 0.78 |
| C4 | 30 | F | 113 | Yes | Positive | 6.43 | Positive | 39.30 | Negative | 0.45 |
| C5 | 33 | F | 205 | Yes | Positive | 7.54 | Negative | 12.83 | NA | NA |
| C6 | 38 | M | 205 | Yes | Positive | 16.02 | Positive | 41.39 | NA | NA |
| C7 | 39 | F | 122 | Yes | Positive | 16.99 | Positive | 40.38 | Borderline | 0.89 |
| C8 | 40 | F | 111 | Yes | Negative | 0.30 | Negative | 11.74 | NA | NA |
| C9 | 44 | F | 122 | Yes | Positive | 16.12 | Positive | 42.58 | Negative | 0.50 |
| C10 | 45 | F | 206 | Yes | Positive | 17.12 | Positive | 129.27 | NA | NA |
| C11 | 48 | F | 137 | Yes | Positive | 1.63 | Negative | 11.10 | Negative | 0.28 |
| C12 | 48 | F | 205 | Yes | Positive | 17.34 | Positive | 249.19 | NA | NA |
| C13 | 50 | F | 95 | Yes | Positive | 11.15 | Negative | 17.64 | Borderline | 0.96 |
| C14 | 51 | F | 205 | Yes | Positive | 17.87 | Positive | 154.11 | NA | NA |
| C15 | 54 | M | 120 | Yes | Positive | 1.38 | Borderline | 25.59 | Negative | 0.43 |
| C16 | 58 | M | 205 | Yes | Positive | 18.29 | Positive | 362.28 | NA | NA |
| C17 | 59 | M | 205 | Yes | Positive | 9.97 | Positive | 44.92 | NA | NA |
| C18 | 60 | F | 205 | Yes | Positive | 17.33 | Positive | 42.17 | NA | NA |
| C19 | 66 | M | 48 | Yes | Positive | 18.43 | Positive | 743.44 | NA | NA |
| C20 | 50 | M | 111 | No | Negative | 0.25 | Negative | 10.40 | NA | NA |
| C21 | 35 | M | 96 | No | Positive | 3.84 | Negative | 11.90 | Negative | 0.40 |
| C22 | 31 | M | 151 | No | Positive | 13.40 | Negative | 15.12 | NA | NA |
| C23 | 50 | M | 97 | No | Positive | 15.03 | Positive | 75.47 | Positive | 1.32 |
| C24 | 23 | M | 99 | No | Positive | 16.31 | Positive | 80.86 | Borderline | 0.92 |
Results for total Ig against SARS-CoV-2 RBD and for IgA against SARS-CoV-2 domain S1 are shown as RoA. For IgG against SARS-CoV-2 domain, S1 results are presented in binding antibody units per milliliter of serum (BAU∙mL−1). Serum samples used for the neutralization experiments are indicated in “NT analysis” column. The average age of the participants was 43 years; the percentage of women was 54%. All samples of convalescent patients were taken between 22 and 206 days after the second dose. NA, Not applicable.
Table E5.
Antibody titers
| Antibody test and group | Median antibody titer (lower, upper 95% CL) |
|---|---|
| IgG | |
| ChAdOx1 | 259.5 (181.3, 337.9) |
| BNT162b2 | 679.0 (626.1, 733.7) |
| mRNA-1273 | 618.6 (492.4, 672.9) |
| Convalescent | 41.8 (17.6, 77.6) |
| IgA | |
| ChAdOx1 | 0.7 (0.6, 1.4) |
| BNT162b2 | 5.3 (3.9, 7.1) |
| mRNA-1273 | 3.9 (0.9, 6.0) |
| Convalescent | 0.6 (0.4, 1.0) |
| Total Ig | |
| ChAdOx1 | 18.4 (17.9, 18.7) |
| BNT162b2 | 18.0 (17.5, 18.7) |
| mRNA-1273 | 18.5 (18.1, 18.7) |
| Convalescent | 15.5 (6.4, 17.0) |
CL, Confidence limit.
Table E6.
Neutralization titers
| Participant group and virus | Median NT50 (lower, upper 95% CL) | Median NT90 (lower, upper 95% CL) |
|---|---|---|
| ChAdOx1 | ||
| WT | 322.7 (260.6, 606.4) | 86.8 (56.0, 109.5) |
| Alpha | 460.9 (291.8, 640.0) | 121.4 (55.6, 240.1) |
| Alpha-E484K | 107.3 (53.1, 224.1) | 28.2 (10.4, 47.8) |
| Beta | 179.6 (137.7, 224.0) | 23.5 (15.4, 68.4) |
| Delta | 64.7 (34.0, 200.8) | 15.0 (9.1, 30.6) |
| BNT162b2 | ||
| WT | 217.5 (128.0, 854.1) | 115.7 (63.6, 301.6) |
| Alpha | 315.2 (164.5, 838.2) | 89.0 (40.8, 271.1) |
| Alpha-E484K | 72.8 (53.2, 348.8) | 11.4 (3.5, 73.2) |
| Beta | 129.2 (64.6, 251.0) | 41.9 (17.4, 181.0) |
| Delta | 214.2 (105.1, 300.1) | 47.9 (21.1, 110.3) |
| mRNA-1273 | ||
| WT | 541.9 (232.1, 1291.0) | 137.8 (62.5, 376.7) |
| Alpha | 996.5 (564.6, 1407.0) | 345.5 (245.4, 504.6) |
| Alpha-E484K | 270.4 (228.3, 596.1) | 78.3 (22.3, 172.0) |
| Beta | 405.7 (83.4, 642.9) | 86.8 (17.1, 170.5) |
| Delta | 466.8 (102.1, 835.4) | 131.1 (24.9, 251.8) |
| Convalescent | ||
| WT | 241.9 (109.1, 353.8) | 45.7 (18.7, 193.1) |
| Alpha | 420.0 (208.5, 769.8) | 82.0 (23.0, 168.0) |
| Alpha-E484K | 56.9 (22.4, 129.8) | 1.0 (1.0, 5.2) |
| Beta | 161.7 (92.4, 308.5) | 12.4 (1.0, 94.9) |
| Delta | 92.0 (3.4, 221.3) | 1.0 (1.0, 16.2) |
CL, Confidence limit.
References
- 1.Sanmarchi F., Golinelli D., Lenzi J., Esposito F., Capodici A., Reno C., et al. Exploring the gap between excess mortality and COVID-19 deaths in 67 countries. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.17359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadoff J., Gray G., Vandebosch A., Cardenas V., Shukarev G., Grinsztejn B., et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against COVID-19. N Engl J Med. 2021;384:2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heath P.T., Galiza E.P., Baxter D.N., Boffito M., Browne D., Burns F., et al. Safety and efficacy of NVX-CoV2373 COVID-19 vaccine. N Engl J Med. 2021;385:1172–1183. doi: 10.1056/NEJMoa2107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., Tukhvatulin A.I., Zubkova O.V., Dzharullaeva A.S., et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Medicines Agency (EMA) EMA considerations on COVID-19 vaccine approval. https://www.ema.europa.eu/en/ema-considerations-COVID-19-vaccine-approval November 11, 2020. Available at:
- 9.US Department of Health and Human Services; US Food and Drug Administration; Center for Biologic Evaluation and Research Development and licensure of vaccines to prevent COVID-19. https://www.fda.gov/media/139638/download June 2020. Available at:
- 10.Plante J.A., Liu Y., Liu J., Xia H., Johnson B.A., Lokugamage K.G., et al. Spike mutation D614G alters SARS-CoV-2 fitness. Nature. 2021;592(7852):116–121. doi: 10.1038/s41586-020-2895-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies N.G., Abbott S., Barnard R.C., Jarvis C.I., Kucharski A.J., Munday J.D., et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021;(6538):372. doi: 10.1126/science.abg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giles B., Meredith P., Robson S., Smith G., Chauhan A., Pacific, et al. The SARS-CoV-2 B.1.1.7 variant and increased clinical severity—the jury is out. Lancet Infect Dis. 2021;21:1213–1214. doi: 10.1016/S1473-3099(21)00356-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheikh A., McMenamin J., Taylor B., Robertson C. Public Health Scotland and the EAVE II Collaborators. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397(10293):2461–2462. doi: 10.1016/S0140-6736(21)01358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisman D.N., Tuite A.R. Progressive increase in virulence of novel SARS-CoV-2 variants in Ontario, Canada. medRxiv. 2021 doi: 10.1503/cmaj.211248. 2021.07.05.21260050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou D., Dejnirattisai W., Supasa P., Liu C., Mentzer A.J., Ginn H.M., et al. Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera. Cell. 2021;184:2348–2361.e6. doi: 10.1016/j.cell.2021.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Supasa P., Zhou D., Dejnirattisai W., Liu C., Mentzer A.J., Ginn H.M., et al. Reduced neutralization of SARS-CoV-2 B.1.1.7 variant by convalescent and vaccine sera. Cell. 2021;184:2201–2211.e7. doi: 10.1016/j.cell.2021.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Z., Schmidt F., Weisblum Y., Muecksch F., Barnes C.O., Finkin S., et al. mRNA vaccine–elicited antibodies to SARS-CoV-2 and circulating variants. Nature. 2021;592(7855):616–622. doi: 10.1038/s41586-021-03324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen X., Tang H., McDanal C., Wagh K., Fischer W., Theiler J., et al. SARS-CoV-2 variant B.1.1.7 is susceptible to neutralizing antibodies elicited by ancestral spike vaccines. Cell Host Microbe. 2021;29:529–539.e3. doi: 10.1016/j.chom.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu K., Werner A.P., Moliva J.I., Koch M., Choi A., Stewart-Jones G.B.E., et al. mRNA-1273 vaccine induces neutralizing antibodies against spike mutants from global SARS-CoV-2 variants. bioRxiv. 2021 [Google Scholar]
- 20.Xie X., Liu Y., Liu J., Zhang X., Zou J., Fontes-Garfias C.R., et al. Neutralization of SARS-CoV-2 spike 69/70 deletion, E484K and N501Y variants by BNT162b2 vaccine–elicited sera. Nat Med. 2021;27:620–621. doi: 10.1038/s41591-021-01270-4. [DOI] [PubMed] [Google Scholar]
- 21.Wise J. COVID-19: the E484K mutation and the risks it poses. BMJ. 2021;372:n359. doi: 10.1136/bmj.n359. [DOI] [PubMed] [Google Scholar]
- 22.Çölkesen F., Kandemir B., Arslan Ş., Çölkesen F., Yıldız E., Korkmaz C., et al. Relationship between selective IgA deficiency and COVID-19 prognosis. Jpn J Infect Dis. 2021 doi: 10.7883/yoken.JJID.2021.281. [DOI] [PubMed] [Google Scholar]
- 23.Wei J., Stoesser N., Matthews P.C., Ayoubkhani D., Studley R., Bell I., et al. Antibody responses to SARS-CoV-2 vaccines in 45,965 adults from the general population of the United Kingdom. Nat Microbiol. 2021;6:1140–1149. doi: 10.1038/s41564-021-00947-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steensels D., Pierlet N., Penders J., Mesotten D., Heylen L. Comparison of SARS-CoV-2 antibody response following vaccination with BNT162b2 and mRNA-1273. JAMA. 2021 doi: 10.1001/jama.2021.15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bošnjak B., Stein S.C., Willenzon S., Cordes A.K., Puppe W., Bernhardt G., et al. Low serum neutralizing anti–SARS-CoV-2 S antibody levels in mildly affected COVID-19 convalescent patients revealed by two different detection methods. Cell Mol Immunol. 2021;18:936–944. doi: 10.1038/s41423-020-00573-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russell M.W., Moldoveanu Z., Ogra P.L., Mestecky J. Mucosal immunity in COVID-19: a neglected but critical aspect of SARS-CoV-2 infection. Front Immunol. 2020;11(3221) doi: 10.3389/fimmu.2020.611337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cervia C., Nilsson J., Zurbuchen Y., Valaperti A., Schreiner J., Wolfensberger A., et al. Systemic and mucosal antibody responses specific to SARS-CoV-2 during mild versus severe COVID-19. J Allergy Clin Immunol. 2021;147:545–557.e9. doi: 10.1016/j.jaci.2020.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sterlin D., Mathian A., Miyara M., Mohr A., Anna F., Claer L., et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci Transl Med. 2021;13(577) doi: 10.1126/scitranslmed.abd2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ketas T.J., Chaturbhuj D., Cruz-Portillo V.M., Francomano E., Golden E., Chandrasekhar S., et al. Antibody responses to SARS-CoV-2 mRNA vaccines are detectable in saliva. bioRxiv. 2021 doi: 10.20411/pai.v6i1.441. 2021.03.11.434841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheikh-Mohamed S., Isho B., Chao G.Y.C., Zuo M., Nahass G.R., Salomon-Shulman R.E., et al. A mucosal antibody response is induced by intra-muscular SARS-CoV-2 mRNA vaccination. medRxiv. 2021 2021.08.01.21261297. [Google Scholar]
- 31.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 32.Rubio-Acero R., Castelletti N., Fingerle V., Olbrich L., Bakuli A., Wölfel R., et al. In search of the SARS-CoV-2 protection correlate: head-to-head comparison of two quantitative S1 assays in pre-characterized oligo-/asymptomatic patients. Infect Dis Ther. 2021;10:1505–1518. doi: 10.1007/s40121-021-00475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Firestone M.J., Lorentz A.J., Meyer S., Wang X., Como-Sabetti K., Vetter S., et al. First identified cases of SARS-CoV-2 variant P.1 in the United States—Minnesota, January 2021. MMWR Morb Mortal Wkly Rep. 2021;70:346–347. doi: 10.15585/mmwr.mm7010e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tegally H., Wilkinson E., Giovanetti M., Iranzadeh A., Fonseca V., Giandhari J., et al. Detection of a SARS-CoV-2 variant of concern in South Africa. Nature. 2021;592(7854):438–443. doi: 10.1038/s41586-021-03402-9. [DOI] [PubMed] [Google Scholar]
- 35.Lopez Bernal J., Andrews N., Gower C., Gallagher E., Simmons R., Thelwall S., et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puranik A., Lenehan P.J., Silvert E., Niesen M.J.M., Corchado-Garcia J., O’Horo J.C., et al. Comparison of two highly-effective mRNA vaccines for COVID-19 during periods of Alpha and Delta variant prevalence. medRxiv. 2021 [Google Scholar]
- 37.Tang P., Hasan M.R., Chemaitelly H., Yassine H.M., Benslimane F.M., Khatib H.A.A., et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the Delta (B.1.617.2) variant in Qatar. medRxiv. 2021 doi: 10.1038/s41591-021-01583-4. 2021.08.11.21261885. [DOI] [PubMed] [Google Scholar]
- 38.Lafon E., Diem G., Witting C., Zaderer V., Bellmann-Weiler R.M., Reindl M., et al. Potent SARS-CoV-2–specific T cell immunity and low anaphylatoxin levels correlate with mild disease progression in COVID-19 patients. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.684014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184:861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sahin U., Muik A., Vogler I., Derhovanessian E., Kranz L.M., Vormehr M., et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature. 2021;595(7868):572–577. doi: 10.1038/s41586-021-03653-6. [DOI] [PubMed] [Google Scholar]
- 41.Klasse P.J., Nixon D.F., Moore J.P. Immunogenicity of clinically relevant SARS-CoV-2 vaccines in nonhuman primates and humans. Sci Adv. 2021;7 doi: 10.1126/sciadv.abe8065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McMahan K., Yu J., Mercado N.B., Loos C., Tostanoski L.H., Chandrashekar A., et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2021;590(7847):630–634. doi: 10.1038/s41586-020-03041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586(7830):516–527. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- 44.Lavelle E.C., Ward R.W. Mucosal vaccines—fortifying the frontiers. Nat Rev Immunol. 2021 Jul 26 doi: 10.1038/s41577-021-00583-2. Erratum in: Nat Rev Immunol 2021 Aug 3. [DOI] [PMC free article] [PubMed] [Google Scholar]