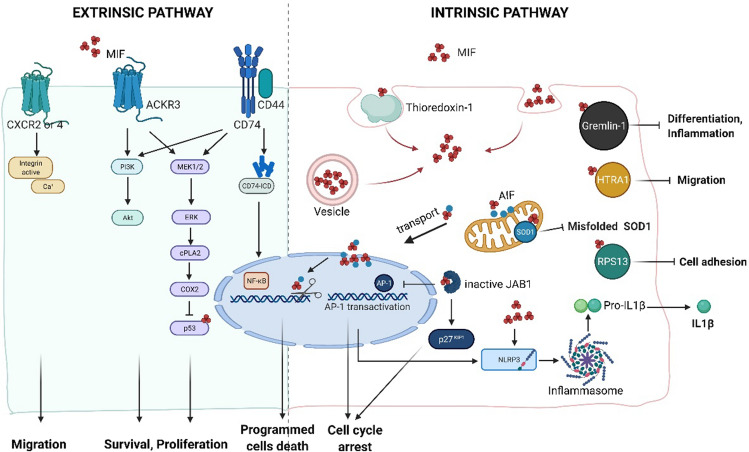
Fig. 2.
MIF-induced signaling pathways (Created with BioRender.com). MIF mediates its biological activities either via membrane receptors (left panel) or through intracellular binding partners (right panel). Extrinsic pathway MIF binds to its membrane receptor CD74 or/and atypical chemokine receptor 3 (ACKR3), which initiates a phosphorylation signal through activation of the mitogen-activated protein kinase (MAPK) and the phosphoinositide 3-kinase (PI3K) pathway. The downstream effects of MIF increase cellular survival and proliferation through the inhibition of p53. The intracellular domain (ICD) of CD74 could enter into nucleus and signal through nuclear factor-κB (NF-κB). The interaction of MIF-CXCR2/4 induces cell migration. Intrinsic pathway Depending on the oxidative environment, cytosolic MIF interacts with thioredoxin-1, resulting in internalization of MIF. Intracellular MIF binds to superoxide dismutase 1 (SOD1) and inhibits the accumulation of misfolded SOD1, protecting from motoneuron damage. Under ischemic or excitotoxic stress, apoptosis-inducing factor (AIF) binds to MIF and guides MIF into the nucleus. Nuclear MIF works as a nuclease and causes DNA damage, leading to cell death. Intracellular MIF interacts with C-Jun activation domain-binding protein-1 (JAB1), inhibiting JAB1-induced transcription of AP-1 and degradation of cyclin-dependent kinase inhibitor 1B (p27Kip1), resulting in cell cycle arrest. Upon damage or infection, intracellular MIF interacts with nitrogen permease regulator-like 3 (NLRP3) and facilitates the interaction between NLRP3 and vimentin, resulting in IL1β release. MIF has been identified as the first endogenous inhibitor of HTRA1, which prevents the inhibition of astrocyte migration. Gremlin-1 also binds to MIF with high affinity, which results in MIF-dependent inflammation and cell differentiation