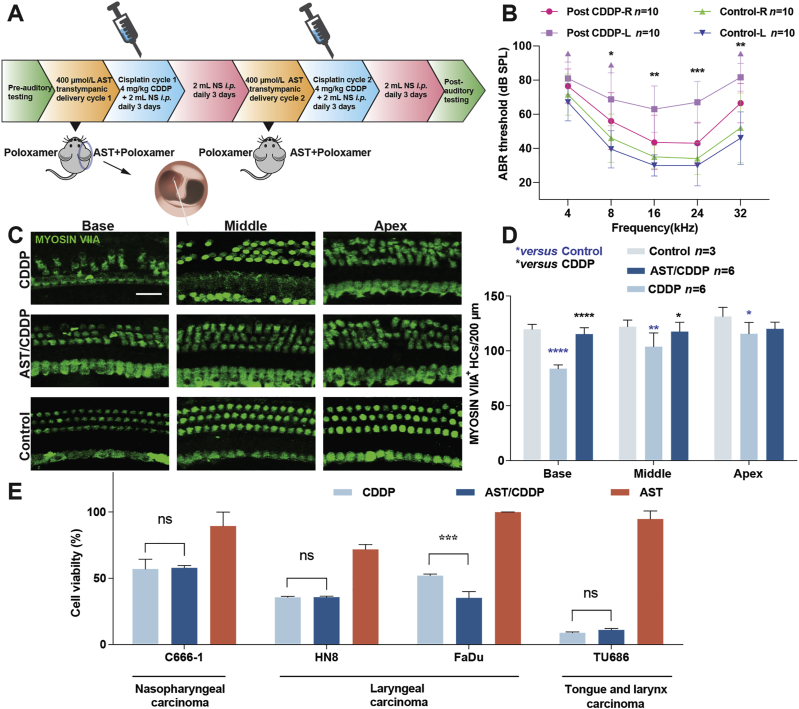
Figure 7.
Astaxanthine protects against cisplatin-induced hearing loss in adult mice and does not compromise cisplatin anti-cancer efficacy. (A) Protocol of administration of astaxanthine and cisplatin into postnatal 45-day FVB mice. (B) ABR threshold shifts following protocol from (A). Both left and right ears from the control groups belong to the same mouse. Left and right ears from the same cisplatin-treated mice delivered with the carrier-only (30% poloxamer) and astaxanthine, respectively. Data are shown as mean ± SD, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001 by two-way ANOVA with Bonferroni correction, n = 10 for each group. (C) and (D) Representative Myosin VIIA (green) stained confocal images of postnatal 45-day FVB mouse cochlear explants from the control, CDDP, and AST/CDDP groups. The numbers of HCs per 200 μm in various cochlear regions were counted by Myosin VIIA staining. Data are shown as mean ± SD, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗∗P < 0.0001 compared with control (blue) and CDDP (black) by two-way ANOVA with Bonferroni correction. Scale bar = 20 μm. (E) Cell survival of multiple head and neck carcinoma cell lines treated with combination of astaxanthine and cisplatin for 48 h. Purple arrow in B indicates no ABR response at the highest stimulus level tested (90 dB). Data are presented as mean ± SD, ∗P < 0.05, ∗∗∗P < 0.001 compared with cisplatin only by one-way ANOVA with Bonferroni correction (n = 3 for each group). ns, not significant; AST, astaxanthine; CDDP, cisplatin; Base, basal turn; Middle, middle turn; Apex, apical turn; NS, normal saline; i.p., intraperitoneal injection.