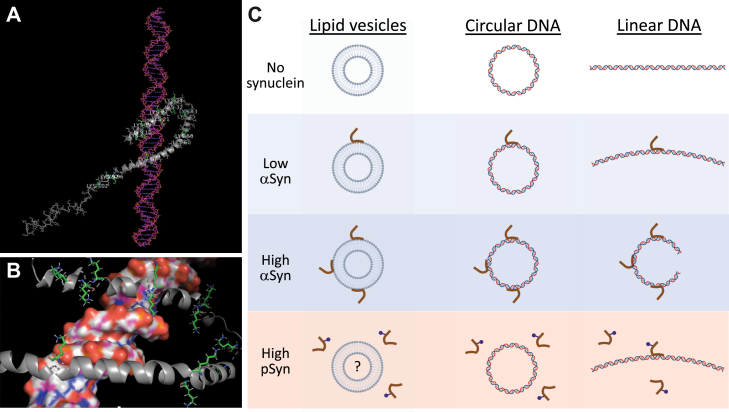
Figure 8.
Model of alpha-synuclein binding to double-stranded DNA and the potential similarities between this DNA binding and binding to phospholipid vesicles.A, single image from an animation (see Fig. S7 for full animation) showing a model of aSyn bound to DNA. The aSyn coordinates come from previous work showing its structure bound to a phospholipid micelle (40). The two curved alpha-helices of the N-terminal domain of aSyn fit into consecutive major grooves of B form DNA. Lysine residues within the protein are labeled. The C-terminal domain, containing the serine-129 phosphorylation site, is poorly resolved in previous structural work, so it is not clear how this phosphorylation may modulate DNA binding. B, single image from an animation (see Fig. S8 for full animation) showing a model of aSyn bound to DNA with 12 lysine residues labeled, using the same structural coordinates as in (A). In this particular model, potential electrostatic interactions between lysine-21, -23, and -80 with the phosphate backbone of DNA are present. C, left, ∼30 nm (in diameter) phospholipid vesicle shown in the presence of no synuclein, low or high alpha-synuclein (aSyn), and high serine-129 phosphorylated alpha-synuclein (pSyn). At low aSyn concentrations, aSyn binds the vesicle given its relatively high affinity for these structures. At high aSyn concentrations, multiple aSyn molecules are bound. To our knowledge, the effects of serine-129 phosphorylation on the interaction of aSyn with phospholipid bilayer vesicles with a diameter of ∼30 nm have not yet been tested. We speculate that serine-129 phosphorylation may inhibit binding in this context. Middle, 300 bp circular DNA having a diameter of ∼30 nm. At low concentrations, aSyn binds DNA given its high affinity for the curved negatively charged DNA surface (due to the phosphate backbone). At high aSyn concentrations, multiple aSyn molecules are bound, analogous to their binding to phospholipid vesicle membranes of similar geometry (left). Serine-129 phosphorylation inhibits the ability of synuclein to bind 300 bp circular DNA. Right, 300 bp linear DNA having a length of ∼100 nm. At low aSyn concentrations, aSyn binds as a monomer and bends linear DNA. At high aSyn concentrations, multiple aSyn molecules bind linear DNA and bend it into a conformation that resembles a DNA circle (middle). At high pSyn concentrations, pSyn binds as a monomer and bends linear DNA, but is not able to bend it into a circle-like form.