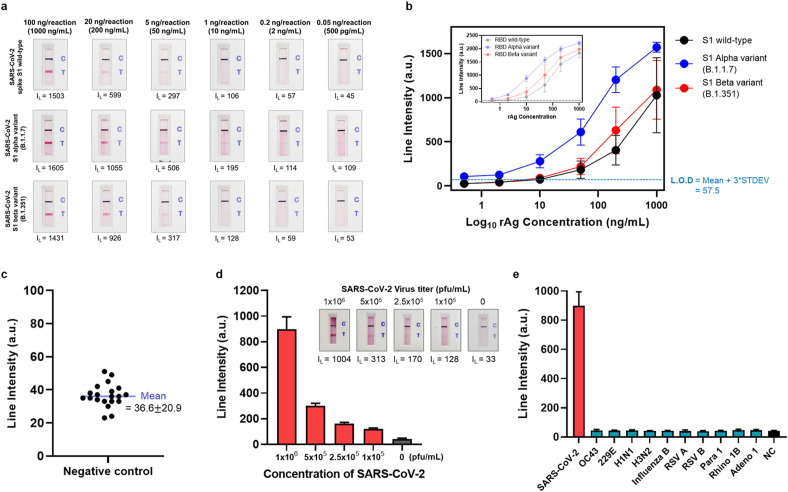
Fig. 3.
Sensitivity and specificity of the ACE2-based biosensor a) Sensitivity of the ACE2-based biosensor for the wild-type SARS-CoV-2 S1 antigen and the alpha and beta variants. Serially diluted samples containing S1 wild-type, S1 alpha variant, and S1 beta variant (concentration: 1000 ng/mL–500 pg/mL) were tested. Twenty minutes after sample loading, the test and control lines were photographed with a smartphone, and the line intensities were measured by a portable line analyzer. b) Sensitivity analysis results for the wild-type SARS-CoV-2 S1 antigen (black line) and the alpha (blue line) and beta variants (red line). Inset) Sensitivity analysis results for the SARS-CoV-2 RBD. The limit of detection (L.O.D) is determined as the mean value of the negative control plus three times the standard deviation. c) The line intensity of 20 negative control samples was also measured. The negative control samples do not contain target antigen (i.e., buffer only). d) Results of sensitivity analysis of live SARS-CoV-2. The live virus was serially diluted (106 pfu/mL–105 pfu/mL) and tested. e) Specificity analysis using human coronaviruses (OC32 and 229E), and other respiratory viruses [human influenza A virus H1N1 (H1N1), human influenza A virus H3N2 (H3N2), human influenza B virus (Influenza B), human respiratory syncytial virus A (RSV A), human respiratory syncytial virus B (RSV B), human parainfluenza virus 1 (Para 1), human rhinovirus 1B (Rhino 1B), and human adenovirus 1 (Adeno 1)].