Abstract
Scientific background
Environmental sampling of SARS-CoV-2 is a fundamental tool for evaluating the effectiveness of non-specific prophylaxis measures in counteracting virus spread. The purpose of our work was to evaluate the effectiveness of the different sampling methods in the hospital setting to assess their correlation with the structural, functional, and operational situation of the monitored departments and to define the dynamics of the spread of the virus in indoor environments.
Methods
The monitoring (air bubbling sampling, surface wipe test) was carried out at the San Martino Polyclinic Hospital (Genoa, Italy) in the period since April 2020 to June 2021. The presence of viral RNA in the collected samples was evaluated by qPCR. The infection capacity of the samples collected was also evaluated by an in vitro challenge test on cells sensitive to SARS-CoV-2 infection.
Results
The percentage of positivity with respect to the number of tests performed (sensitivity) were air bubbler 50%, wipe test 17%, and challenge test 11%. Only 20% of the samples tested positive in the wipe test and 43% of the samples tested positive in the bubbler sampling were also positive in the challenge test. All the positivity obtained was detected at a distance of less than 2 m and height of less than 1.5 from COVID-19 patients.
Conclusions
Environmental contamination from SARS-CoV-2 detected at the San Martino Polyclinic Hospital is found lower than similar assessments performed in other hospitals both in Italy and abroad. Our study predicted that environmental monitoring of SARS-CoV-2 must be carried out in an integrated way by not using a single sampling method, as each individual test has a different biological significance and performance. However, the virus detected by wipe test only is often a degraded viral fragment and not an intact infecting virion.
Keywords: Hospital contamination, SARS-CoV-2, Environmental monitoring, Challenge test, Viral RNA
1. Introduction
SARS-CoV-2 is mainly transmitted through infected respiratory droplets and close contact with the infected person (Riou and Althaus, 2020). Furthermore, there is a risk for aerosol transmission when the virus is exposed to high concentrations of aerosol for a long time in a relatively closed environment. SARS-CoV-2 virus is highly contagious, and people are severely susceptible to it. Many healthcare workers have been infected during patient care during this pandemic (Wang et al., 2020). Environmental sampling of SARS-CoV-2 is a fundamental tool for the prevention of the COVID-19 infection. For this reason, the environmnetal sampling, that allows to define the spreading characteristics of the virus, guides the measures of non-specific environmental prophylaxis. In addition, environmental sampling makes it possible to identify the effectiveness of the prophylaxis and disinfection measures implemented to hold SARS-CoV-2 contagion in confined environments (van Doremalen et al., 2020). WHO defines droplets and droplet nuclei of more than 5 μm in diameter as respiratory aerosols and the residue of up to 5 μm in diameter as dried respiratory aerosols, produced by the evaporation of droplets coughed or sneezed into the atmosphere or aerosolized infective material, respectively (WHO, 2014). Liu et al. reported that the peak concentration of SARS-CoV-2 aerosols appears in two distinct size ranges: at the submicron scale with dominant aerodynamic diameter between 0.25 and 1.0 μm; and at the supermicron scale with diameter greater than 2.5 μm (Liu Y, 2020). The main sources of SARS-CoV-2 aerosols are coughs and sneezes by infected people. The capacity for droplets to travel long distances in airflow is determined largely by their size. (Kampf et al., 2020).
The environmental sampling can be carried out in the following ways: (a) wipe test; (b) sampling of the airborne viral suspension (Becker et al., 2019). The wipe test allows a cumulative assessment of the viral load that is deposited over time with reference especially to that carried by the large aerosol (droplet); it is not quantitative sampling. The sampling of the air diffuse suspension allows the quantitative evaluation of the viral load per cubic meter of sampled air; reflects the viral load also present in the small aerosol which by its nature tends to settle much more slowly over time than in the large aerosol. For this reason, the small aerosol spreads at a much greater distance than the large aerosol with respect to the source of entry (patient or infected person) (Ong et al., 2020). Sampling of the airborne viral suspension is carried out using air-flow sampling pumps. The uptake trap can be liquid (bubbler sampling) or solid with negative ionic charge membrane to selectively capture the SARS-CoV-2 spike proteins characterized by a strong positive electric charge due to the presence of sulfur amino acids (membrane sampling).To the best of our knowledge, IRCCS San Martino is the only hospital in Italy to have carried out structured environmental monitoring of its hospitalization environment during the pandemic by multiple methods to assess the wellbeing and safety of operators and patients. Virus detection on the sampled material (swab wipe test, bubbling liquid, negatively charged membrane) is performed by qPCR. The strong limitation of this approach is the detection of only one component of the virus and that is its RNA. The presence of RNA does not necessarily correspond to the presence of whole virions capable of infecting the subjects which determines the monitored environment. However, the RNA can represent a degradation product of the virus by degrading through physical and chemical environmental agents.
The purpose of our work was to evaluate the effectiveness of environmental sampling for SARS-CoV-2 in the hospital setting to compare and integrate the information provided by different sampling methods. The results obtained were used to evaluate the correlation between environmental sampling and the structural, functional, and operational situation of the monitored departments.
The specific aim of our work was to develop a complementary environmental sampling method for SARS-CoV-2 aimed at demonstrating the presence of whole virions and evaluating their ability to infect sensitive cells. The information on the real biological activity of the virus does not necessarily correspond to the presence of RNA alone. Our work compared the results obtained by the evaluation of RNA and the infecting capacity of the virions detected in the monitored environment. It was thus possible to carry out accurate assessment on the environmental risk of contagion from SARS-CoV-2 in the monitored environment.
2. Materials and methods
2.1. Environmental sampling
Environmental sampling was performed at the IRCCS San Martino Polyclinic Hospital. This facility is the regional referral hub for the treatment of COVID-19 patients. It is therefore suitable for assuring the definite presence of high environmental viral load of SARS-CoV-2. This sampling was performed at the facilities, (a) Emergency Department for COVID-19 patients, third floor laboratory building; (b) Emergency and Acceptance Department (DEA) in the First Aid building on the first floor; (c) Clinic for Infectious Diseases Complex Pathologies building.
Sampling at the Emergency Department was performed in April 2020 by wipe test on the following 12 environmental surfaces: (1) computer keyboard; (2) telephone keypad; (3) patient storage trolley; (4) patient bed rails; (5) patient bed pillow; (6) respiratory gas detector monitors; (7) vertical wall edges; (8) horizontal wall edges; (9) heart rate monitor; (10) drug cart; (11) floor plinths; (12) internal CPAP.
The sampling at the DEA was performed in January, March, April, and May 2021 both by wipe test and bubbling sampling. The wipe test was performed in close proximity to the patients (bedside table, dining table). Bubbling sampling was performed near (distance <2 m), far distance> 3 m) from the patients and at different heights (low 1.5 m, high> 2.5 m). In addition, the bubbling sampling was carried out near the intake openings of the ventilation systems to evaluate the possibility of spreading the virus through them. However, it should be emphasized that this risk can be neutralized, by avoiding the mixing of extracted and injected air. Sampling at the Infectious Diseases Clinic was performed in 2 different rooms in June 2021. Both wipe testing and bubbling sampling were performed in these rooms.
2.2. Wipe test
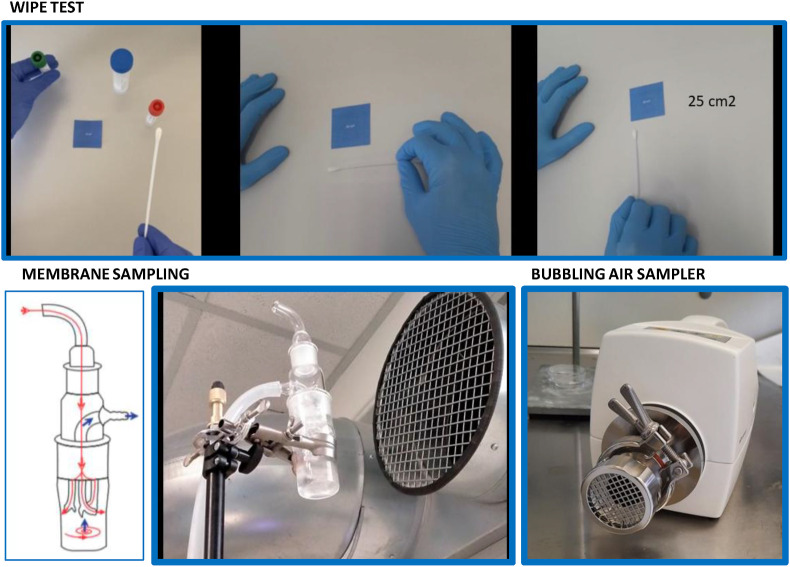
The sampling kit of the company Biocomma (Hong Kong, China) was used, validated by preliminary analysis carried out in collaboration with Department of Life Sciences (DISTAV), University of Genoa. A standardized surface area of 20 cm2 was wiped using a stick embedded with porous fibers at the terminal end. The stick was immersed into the buffer solution and mixed for 30 s.
A total of 29 wipe tests have been performed, 21 in DEA and 8 in Clinic for Infectious Diseases.
2.3. Bubbler sampling
The Air Cube Com 2-TH sampler provided out of courtesy by the Gadomed company (Genoa, Italy), was used. The instrument was equipped with a peristaltic pump collecting air at the standardized flux of 20 m3 per hour. The air was bubbled onto a buffer contained into a glass ampoule. Air collection was performed for 1 h.
A total of 14 bubbler sampling tests have been performed, 12 in DEA and 2 in Clinic for Infectious Diseases.
2.4. Membrane sampling
The MD8 Airport sampler (Sartorius, Rome, Italy) equipped with gelatin filters made available by the Liguria Region, was used. The sampling was performed with an air flow of 20 l/min for 50 min, each using membrane adsorption as an analyte capturing tool (SARS-CoV-2). This membrane (gelatin filter) at the end of the process was removed from the instrument using sterile disposable tweezers and solubilized in a tube containing 2 ml of DMEM enriched in penicillin/streptomycin/ciproxin. (Fig. 1 ).
Fig. 1.
Environmental sampling methods: wipe test, membrane sampling and bubbling air sampler.
A total of 4 membrane sampling tests have been performed, all of them in Clinic for Infectious Diseases.
2.5. Evaluation of the infectious capacity of SARS-CoV-2 by challenge test
The Vero cell challenge test was used. These cells express the ACE2 receptor which represents the binding site of the SARS-CoV-2 spike protein to infect target cells. The environmental sample as such (bubbling liquid) or resuspended (wipe test buffer, thawed membrane) was incubated with Vero cells for 12 h. The resuspension liquid consisted of DMEM along with Hepes and fetal calf serum. At the end of the incubation, the virus was inactivated by heating at 56 °C for 30 min. This heating also involved the loss of adhesion of the cells which entered the suspension and collected and washed by centrifugation. The cell pellet was then collected and subjected to PCR to evaluate the presence of the virus inside the cells.
All procedures were performed at the Biosafety Level 3 (BSL3) laboratory of the IRCCS San Martino Hospital for research on SARS-CoV-2 operating at Pad 90.
2.6. Evaluation of the presence of viral RNA by qPCR
The presence of viral RNA within Vero cells was evaluated by qPCR using the SARS-CoV-2 RT-qPCR Reagent Kit (PerkinElmer, Wathman, MA, USA). The samples were prepared for RNA extraction in an automated robotic station (Janus G3, PerkinElmer, Wathman, MA, USA). The samples, composed of Vero cells resuspended in physiological solution (300 μl) were mixed with a solution containing poly (A) RNA buffer and proteinase K (14 μl). The RNA extraction was carried out using the Chemagic automated station and the related magnetic ball extraction kit (PerkinElmer, Wathman, MA, USA). For each assay, 3 Taqman qPCR probes were used for (a) house-keeping gene (Ribonuclease P/MRP Subunit P30 [RPP30] used as internal control; (b) SARS-CoV-2 Orf1ab viral gene (Vic labeled); and (c) SARS-CoV-2 N viral gene (FAM labeled). The purified RNA was subjected to PCR amplification cycles according to the following parameters: 50 °C × 15 min, 95 °C × 2 min, 45 cycles at 95 °C × 3 s, and 60 °C. × 30 s. The PCR reaction was performed in a final volume of 20 μl using the Light Cycler 480II (Roche) automated robotic device.
2.7. Positive reference samples
Two types of positive reference samples were used: (a) pool of anonymous pharyngeal tampons collected from patients with molecular qPCR diagnosis of the presence of SARS-CoV-2 virus with cycle positivity ≤25; (b) generation of environmental aerosols starting from the sample just described. The Pro Pharma RF7 device was used to generate the aerosol. 5 ml of sample was used to generate nebulized aerosol for 1 h. The generated aerosol was conveyed through flexible plastic tubes to the bubbler sampler. The system was set up under a biosecurity hood in closed mode to avoid accidental spreading of the virus into the environment.
2.8. Statistical analysis
Descriptive statistics was used to present data as numbers and percentages. The differences in the positive rates between the sampling methods were compared by Fisher exact tests. The numbers of sample locations were calculated based on the area of each room in accordance with the ISO 14644-1. Statistical analysis was performed with SPSS Statistics version 25 (IBM, SPSS Inc., Chicago, IL, USA).
3. Results
A total 29 swab samples were collected within the twelve sampling sites at the Emergency Department, and their environmental monitoring data by wipe test is shown in Table 1 . PCR results of environmental monitoring of SARS-CoV-2 in the DEA Department by PCR were negative (Table 2 ). The positivity rate was higher in contaminated air sampling site close (<1 m) to the patients. Positivity PCR cycle rate of 39.2 was observed as a result of bubbler sampling (Table 3 ). The positive rates of swab samples from environmental surfaces of specific sites regardless of area at the Emergency Department are detailed in Table 4 . Challenge test was positive with 35.1 rate at close air (50 cm) to the patients. 7 positive patients were present in the room where the samples were taken, and the mechanical ventilation was missing (Table 4). Results of environmental monitoring of SARS-CoV-2 in the DEA Department by bubbler were positive for PCR test to 37.5 in Air near (50 cm) to the patients at bed of 80 cm height. (Table 5 ). Results of environmental monitoring in Infectious Diseases Department are reported in Table 6, Table 7 . The positive rate was 20% with wipe test, and 43% with bubbler sampling. The higher positivity rate was measured by challenge test, near (<1 m) from patients at 0.6 m height as compared to the bubbler sampling.
Table 1.
Results of the environmental monitoring of SARS-CoV-2 in the Emergency Department April 2020.
| Sampling site | Sampling method | PCR | PCR positivity cycle | PCR challenge test |
|---|---|---|---|---|
| computer keyboard | Wipe | negative | >40 | NT |
| telephone keypad | Wipe | negative | >40 | NT |
| patient storage trolley | Wipe | negative | >40 | NT |
| patient bed rails | Wipe | negative | >40 | NT |
| patient bed pillow | Wipe | negative | >40 | NT |
| respiratory gas detector monitors | Wipe | negative | >40 | NT |
| horizontal wall edges | Wipe | negative | >40 | NT |
| heart rate monitor | Wipe | negative | >40 | NT |
| medication cart | Wipe | negative | >40 | NT |
| floor plinths | Wipe | negative | >40 | NT |
| internal CPAP | Wipe | positive | 21 | NT |
NT, not tested.
Table 2.
Results of environmental monitoring of SARS-CoV-2 in the DEA Department in January 2021.
| Sampling site | Sampling method | PCR | PCR positivity cycle | PCR challenge test |
|---|---|---|---|---|
| patient bedside table | Wipe | negative | >40 | negative |
| patients dining table | Wipe | negative | >40 | negative |
| Close air (<2 m) at patients at 1.5 m of height | bubbler sampling | negative | >40 | negative |
| Far air (>3 m) at patients at 1.5 m of height | bubbler sampling | negative | >40 | negative |
| Air at a height of 2.5 m near the aeraulic system intake vent | bubbler sampling | negative | >40 | negative |
NT, not tested.
Table 3.
Results of environmental monitoring of SARS-CoV-2 in the DEA Department in March 2021.
| Sampling site | Sampling method | PCR | PCR positivity cycle | PCR challenge test |
|---|---|---|---|---|
| Close air (<1 m) at patients at 1.5 m of height for 1 h | bubbler sampling | positive | 39.2 | negative |
| Close air (<1 m) at patients at 1.5 m of height for 3 h | bubbler sampling | negative | >40 | negative |
| Far air (>3 m) at patients at 1.5 m of height for 1 h | bubbler sampling | negative | >40 | negative |
| Far air (>3 m) at patients at 1.5 m of height for 3 h | bubbler sampling | negative | >40 | negative |
NT, not tested.
Notes: Boundary conditions on the day of sampling 25-03-2021.
- Positive patients present in the ward: 29.
- Positive patients present in the open space where the sampling took place: 7.
- Volume of the open space: 180 m3
- Air volume per patient: 25.7 m3
- Indoor thermohygrometric conditions: T 22.9 °C - R.H. 40.9%.
- Air flow extracted from the vent installed in the open space: 2465 m3/h.
- Hourly extraction volumes: 13.65.
Table 4.
Results of environmental monitoring of SARS-CoV-2 in the DEA Department in April 2021.
| Sampling site | Sampling method | PCR | PCR positivity cycle | PCR challenge test |
|---|---|---|---|---|
| Patients bed side table | Wipe | positive | 26.4 | positive (37.3) |
| patients dining table | Wipe | positive | 37.6 | negative |
| patients service table | Wipe | positive | 38.2 | negative |
| table away from patients | Wipe | negative | >40 | negative |
| Close air (<50 cm) at patients at 1.5 m of height | bubbler sampling | positive | 25.2 | positive (35.1) |
| Far air (>2 m) at patients at 1.5 m of height | bubbler sampling | positive | 36.6 | negative |
| Distant air (>2 m) at a height of 2.5 m near the air intake vent | bubbler sampling | negative | >40 | negative |
NT, not tested.
Notes: Boundary conditions on the day of sampling 21-04-2021.
- Positive patients present in the ward: 13.
- Positive patients present in the room where the samples were taken: 7.
- Volume of the room: 165 m3
- Air volume per patient: 24 m3
- Indoor thermohygrometric conditions: T 22.8 °C - R.H. 49.8%.
- No mechanical ventilation.
- Natural ventilation estimated at 0.5 vol/h.
Table 5.
Results of the environmental monitoring of SARS-CoV-2 in the DEA Department in patient service table.
| Sampling site | Sampling method | PCR | PCR positivity cycle | PCR challenge test |
|---|---|---|---|---|
| patient bedside table | Wipe | negative | >40 | negative (>40) |
| patients dining table | Wipe | negative | >40 | negative (>40) |
| patient service table | Wipe | negative | >40 | negative (>40) |
| armrest chair located near the patient | Wipe | negative | >40 | negative (>40) |
| Air close (50 cm) to patients at 80 cm high Bed | bubbler sampling | positive | 36.5 | positive (37.5) |
| Air close (50 cm) to patients at 80 cm high Bed | bubbler sampling | NT | NT | NT |
NT, not tested.
Table 6.
Results of environmental monitoring of SARS-CoV-2 in Infectious Diseases Department on June 4, 2021.
| Sampling site | Sampling method | PCR | PCR positivity cycle | PCR challenge test |
|---|---|---|---|---|
| Room 1 | ||||
| patient bedside table | Wipe | positive | 28.7 | Negative (>40) |
| patients dining table | Wipe | negative | >40 | Negative (>40) |
| Near air (<1 m) from patients at 0.6 m height | bubbler sampling | positive | 36.8 | Positive (37.4) |
| Room 2 | ||||
| patient bedside table | Wipe | negative | >40 | Negative (>40) |
| patients dining table | Wipe | negative | >40 | Negative (>40) |
| Near air (<2 m) from patients at 1.5 m height | bubbler sampling | positive | 32.4 | Negative (>40) |
NT, not tested.
Negative = positivity cycle PCR >40.
Table 7.
Results of the environmental monitoring of SARS-CoV-2 in the Infectious Diseases Department on June 23, 2021.
| Sampling site | Sampling method | PCR | PCR challenge test |
|---|---|---|---|
| Room 1 | |||
| patient bedside table | Wipe | negative | negative |
| Patient dining table | Wipe | negative | negative |
| Near air (<1 m) from patients at 0.6 m height | Membrane sampling | negative | negative |
| Far air (>3 m) at patients at 1.5 m of height | Membrane sampling | negative | negative |
| Room 2 | |||
| patient bedside table | Wipe | negative | negative |
| dining table | Wipe | negative | negative |
| Near air (<2 m) from patients at 1.5 m height | Membrane sampling | negative | negative |
| Far air (>3 m) at patients at 1.5 m of height | Membrane sampling | negative | negative |
NT, not tested.
Negative = positivity cycle PCR ≥40.
The wipe test showed a sensitivity of 17%, and 83% negatives for viral RNA (Table 8 ). The advantage of wipe test was to evaluate the accumulation of the viral load over time on a solid surface. Number of positive samples with confirmed positivity through challenge test, was reported in Table 9 . Overall performance of each SARS-CoV-2 environmental sampling method was reported in Table 8. The positivity rate observed by wipe test was 17% for <1 m from patient, and was 50% by < 2 m height and by < 1 m from patient, detected by bubbler sampling, the positivity rate was 11% measured by challenge test <1 m and height 80 cm and 0.6 m from patient (Table 8).
Table 8.
Overall performance of each SARS-CoV-2 environmental sampling method.
| Number of samples performed | Negatives (%) | Positives (%) | Notes | |
|---|---|---|---|---|
| Wipe test | 29 | 24 (83%) | 5 (17%) | 1°+3°°+1°°° |
| Bubbler sampling | 14 | 7 (50%) | 7 (50%) | 1* + 2**+1*** + 1****+2**** |
| Membrane sampling | 4 | 4 (100%) | 0 (0%) | |
| Challenge test | 35 | 31 (89%) | 4 (11%) | 2§ + 1§§+1§§§ |
| Total | 82 | 66 (80%) | 16 (20%) |
Notes.
° CPAP Rep Emergency.
°° DEA Distance <1 m from patient.
°°° M Inf Distance <1 m from patient.
* DEA Distance <1 m from patient.
** DEA Distance <2 m height 1.5 m (negative at height 2.5 m).
*** DEA Distance <1 m from patient, height 80 cm.
**** DEA <1 m from patient, height 80 cm.
****M Inf <1 m from patients at 0.6 m height; <2 m in patients at 1.5 m of height.
§ Distance <1 m from patient.
§§ <1 m from patient, height 80 cm.
§§§ <1 m from patients at 0.6 m in height.
Table 9.
Number of positive samples whose positivity is also confirmed by the challenge test.
| Number of positive detections | Number of positive findings in the challenge test | |
|---|---|---|
| Wipe test | 5 | 1 (20%) |
| Bubbler sampling | 7 | 3 (43%) |
| Total | 12 | 4 (33%) |
The challenge test was the only one used to directly assess the ability of the virus to penetrate sensitive cells. However, 20% of the samples tested positive in the wipe test were also positive in the challenge test. Schematic diagram showing environmental sampling sites in the inpatient area of the hospital is reported in Fig. 2 .
Fig. 2.
Schematic diagram showing environmental sampling sites in the inpatient area of the hospital. The presence of the virus is indicated in red and the absence in green. Contaminated areas are those close air (<1 m) at patients at 1.5 m of height for 1 h. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
Multiple strategies to mitigate the risks of transmission have been adopted globally, but there is still a paucity of evidence addressing key questions, such as airborne transmissibility, that may be a consequence of the difficulty in analyzing a virus that is very sensitive to small environmental changes (Morawska and Cao, 2020). The obtained results demonstrate that, environmental monitoring of SARS-CoV-2 should be carried out in an integrated manner and not using a single sampling method. Indeed, every single test (wipe, bubbler, membrane, challenge) has different performance and meaning. At this regard, further to those used in the herein presented study, also quantitative test could be proposed exactly quantifying the number of viral genomic copies per cubic meter of sampled air (Robotto et al., 2021).
Our results indicate that the most sensitive test (number of positives compared to the number of tests carried out) is bubbler sampling (sensitivity 50%). This test, together with membrane sampling, allows a quantitative evaluation of the airborne viral load per cubic meter of air. The sample flow is adjustable and well measured. It is therefore possible to indicate the concentration of viruses in the air of the sampled environment. The wipe test showed a sensitivity of 17%. The advantage of this test is to evaluate the accumulation of viral load over time on a solid surface. However, this test has little value in view of unlike contagiousness as compared to the bubbling sampling in which airborne viral load is evaluated. Furthermore, the virus deposited on environmental surfaces exposed to environmental noxae rapidly degrades (light, temperature, low humidity, presence of oxygen) (Biryukov J et al., 2020). SARS-CoV-2, like all viruses, is an intracellular parasite that outside the host enjoys a very short autonomous life, especially if not integrated into coarse biological matrices. The challenge test is the only one among those used to directly evaluate the ability of the virus to penetrate the sensitive cells, thus determining the presence in the analyzed matrix of intact infecting virions. (Binder R.A. et al., 2020). Contrarily, all other tests evaluate merely the presence of viral RNA fragments which, as such, can derive from degraded and fragmented virions, which are no longer able to cause infection. The membrane sampling showed low sensitivity, as it did not detect positive results. This was due to the following factors:
-
(a)
brevity of sampling. The interest of membrane sampling derives from its ability to carry out long-term sampling even longer than 3 h. This possibility does not exist for the bubbling sampler which, due to the progressive evaporation of the bubbling liquid, cannot carry out sampling lasting more than 1 h (as performed in the study presented here). However, in our study the version of membrane sampler available had a battery with a maximum autonomy of 1 h, so it was not possible to extend the sampling for longer times. In addition, the membrane sampler is much louder than the bubbler sampling. This element is of particular importance in the choice of the sampling method in the wards where it is necessary to cause the least possible disturbance to patients and healthcare workers.
-
(b)
low number of samplings performed: 4 compared to 14 of the bubbler sampling;
-
(c)
sampling period: membrane sampling was performed only at the end of June. In this period, the pandemic curve showed a significant decline. Therefore, the number of COVID-19 patients in the wards examined was significantly lower than in the previous months. Furthermore, the airborne viral load emitted by the patients was significantly lower in June than the previous months, as detected by PCR analysis of throat swab. This data was directly available only for the samplings performed at the Infectious Diseases Department where the mean PCR positive cycle of the patients present at the time of sampling was 35.1 ± 3.6 (mean ± standard deviation of 10 analyses). For comparison, in the period January–April of the same year, the average value obtained in the satellite laboratory for the diagnosis of COVID-19 located in our department was around 28.
Only 20% samples tested positive in the wipe test were also positive in the challenge test. This result demonstrates how wipe test significantly overestimates the presence of infecting SARS-CoV-2. In fact, only in a modest percentage of cases the virus detected by wipe tests is not a degraded viral fragment but an intact infecting virion. This situation is different when comparing the challenge test with the bubbler sampling. In this case the percentage of samples collected with a bubbler which are positive at the challenge test also, rises to 43%. This result is justified by the fact that the bubbler sampling collects the airborne viral load emitted by the patient before its deposit on the surface and therefore less subjected to the noxae of environmental degradation of the virus than what happens for the wipe test. It is of particular interest that all the positivity obtained was detected only at distance less than 2 m from the patient and at heights of less than 1.5 m. In only one case a positivity was detected at a distance greater than 1 m and at height greater than 80 cm from the floor. This result indicates that the environmental diffusion capacity of SARS-CoV-2, considering whole infecting virion rather than its degraded fragments, is not particularly strong. Infectious SARS-CoV-2 therefore spreads mainly with the large droplets that fall in the vicinity of the patient and not with the smaller aerosol components of the emitted Flugge by the patient. This situation is consistent with the environmental change of the virus, which is more preserved as whole infecting virion, especially in the presence of coarse biological matrices (large droplets) capable of preserving it from environmental noxae (Otter J.A. et al., 2016). Also, infecting virions have not been detected above 1.5 m in height and specifically in the vicinity of the air intake vents of the aeraulic systems. It therefore appears extremely unlikely that SARS-CoV-2 will spread through such implants unless the suction port is located less than 1.5 m high and less than 2 m from the patient. Therefore, it does not seem appropriate to carry out excessive sanitization procedures of these plants and their closure, which would decrease the air circulation thus decreasing the per capita air cube and increasing the environmental viral load. Furthermore, the decrease in ventilation and air cooling, with the consequent loss of optimization of the indoor environmental temperature, could have negative influences on mortality especially during the summer and increasingly frequent heat wave months (Zhao et al., 2021). Available data indicates that SARS-CoV-2 spreads more rapidly than Severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) coronaviruses and it largely transmits through respiratory droplets during the close contact with infected individuals (Chang et al., 2020). Infection also occurs when people contact with contaminated surfaces (Chan et al., 2020; WHO, 2020; Kim et al., 2016). However, many patients hospitalized with COVID-19 have been placed in airborne infectious isolation rooms with frequent air changes and routine hygiene processes. Recently, two studies reported the surveillance of environmental contamination during the hospitalization of SARS-CoV-2-infected patients, and suggested that surface contamination may be the main virus transmission route in hospitals (Ong et al., 2020; Cheng et al., 2020). Indeed, in hospitals CPAP equipments (when used) shield airborne viral diffusion, as demonstrated by our findings that CPAP was the only site where SARS-CoV-2 was detected by wipe test in the Emergency Department.
The positive results obtained with respect to the number of samplings carried out, which indicate the presence of SARS-CoV-2 in the environments examined, are in line with the findings of other studies in other hospitals both in Italy and abroad. A recent study monitored the presence of environmental SARS-CoV-2 in the San Paolo e Carlo hospital in Milan (Italy) (Razzini et al., 2020). In this study, 42 environmental samples were examined using PCR alone. The percentage of samples positive to the wipe test was found to be 35% in the areas with high contamination and 50% with intermediate contamination. These values are higher than 17% obtained at the San Martino Polyclinic in highly contaminated areas (DEA, Infectious Diseases Department). Furthermore, all (100%) evaluations of airborne viral load in areas of high contamination, carried out with a membrane sampler similar to the one we used, gave positive results. For comparison, the frequency of positive samples at the San Martino Polyclinic, detected by bubbler sampling, was equal to 50%. A multicenter study carried out in South Korea evaluated environmental contamination from SARS-Cov-2 using a membrane sampler, similar to the one we used, on 52 samples and by wipe test on 320 samples (Kim et al., 2020). All the samples collected by membrane sampler were negative, like what we found at the San Martino Polyclinic using the same sampler. 27% of the wipe tests were positive, a value higher than the 17% we observed at the San Martino Polyclinic where only wards with a high risk of contamination were examined. It is interesting to note that this study also identifies the distance from patients as a factor strongly influencing the positivity of the samples. In fact, the possibility of transmission at distances greater than 2 m is defined as remote, similarly to what we have observed.
Another study investigated the environmental presence of SARS-CoV-2 in 45 environmental samples using wipe tests in the University Hospital of Zhejiang (China) during the pandemic peak that occurred in 2019 (Wang et al., 2020). PCR positivity was observed only in black water samples. This positivity was not confirmed by the challenge test. This result confirms that the presence of PCR positive environmental samples does not correspond to the presence of whole infecting virions.
The presence of SARS-CoV-2 in the environment was investigated in the hospital of the National Center for Infectious Diseases in Singapore (China) (Chia et al., 2020). The presence of the virus as an air-diffused feed was evaluated with an aerosol sampler by collecting the particulate matter of dimensions between 1 and 4 μm. The sampling of the surfaces was carried out by wipe test. Sampling of the airborne viral load gave positive results in 2 of the 3 samples analyzed (67%), compared to the 50% observed by us at the high intensity wards of the San Martino Polyclinic in Genoa. It is interesting to note that the positivity observed in this study was all related to the larger particle sizes while the virus was not detectable in the particle sizes with dimensions less than 1 μm and the viral load was proportional to the size of the particle size. This result confirms that SARS-CoV-2 spreads in confined environments especially with the large droplet. As regards the sampling on surfaces carried out by analyzing 245 samples, the most frequent positivity was observed on the floor (65), on the armrest of the bed (59%) and on the bedside table (47%). These last two sites are characterized by a minimum distance from the patient, thus confirming the limited environmental diffusion capacity of the virus. All these values are greater than the positive frequencies observed in the wipe test at the San Martino Polyclinic (17%). Therefore, the environment examined at the San Martino Polyclinic Hospital (Genoa, Italy) appears to be in line with international standards regarding the risk of hospital transmission of SARS-CoV-2. Indeed, the IRCCS San Martino Polyclinic Hospital today represents one of the few principals in Italy that, in collaboration with the Liguria region, has an autonomous environmental monitoring program of SARS-CoV-2. As per our insight and based on the scientific literature analyzed, this Polyclinic is the only health facility that has used an integrated environmental monitoring plan through multiple tests (wipe, bubbler, membrane, challenge) with long-term monitoring of the duration of 2 years (from April 2020 to June 2021). Thus, results obtained from our study indicate that SARS-CoV-2 is strongly affected by environmental noxae which are easily able to neutralize it within 2 m distance from the patient. It is therefore of great interest to evaluate the possibility of further decrease in the airborne viral load in confined spaces using air sanitization devices, in future studies.
5. Conclusion
The study carried out made it possible to develop an innovative and original integrated program for the environmental monitoring of SARS-CoV-2. The most important and original aspect of the results obtained is an integrated monitoring method which is capable of evaluating not only the presence of degraded fragments of the virus but the infecting virions also. The availability of this information appears to be crucial to concretely define the risk of contagion in the confined environment.
Authors’ contribution
Izzotti A. and Tiso M. Conceptualization. Grasselli E., Barbaresi M., Pfeffer U, Bixio M., Colombo M., Methodology. Sossai D., Izzotti A. Borneto A., Boccaccio A., Manfredi V. Data curation. Izzotti A., Grasselli E and Pulliero A. Writing- Original draft preparation. Grasselli E Borneto A., Boccaccio A., Manfredi V., Bassetti M. Visualization. Izzotti A. Nicosia E, Tiso M Investigation. Izzotti A. and Tiso M Supervision. Izzotti A., Grasselli E., and Tiso M Validation. Izzotti A. Pulliero A. Writing- Reviewing and Editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by Regione Liguria Project ‘Evaluation of infectiveness of pathogen viruses in water’ (DR 2020-AC-182, 26/5/2020) and by Gadomed S.r.l. Genoa, Italy.
A thank goes to Zumama Khalid, a PhD student for her help with the English language revision.
References
- Becker B., Henningsen L., Paulmann D., Bischoff B., Todt D., Steinmann E., Steinmann J., Brill F.H.H., Steinmann J. Evaluation of the virucidal efficacy of disinfectant wipes with a test method simulating practical conditions. Antimicrob. Resist. Infect. Control. 2019 Jul 16;8:121. doi: 10.1186/s13756-019-0569-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder R.A., Alarja N.A., Robie E.R., Kochek K.E., Xiu L., Rocha-Melogno L., Abdelgadir A., Goli S.V., Farrell A.S., Coleman K.K., Turner A.L., Lautredou C.C., Lednicky J.A., Lee M.J., Polage C.R., Simmons R.A., Deshusses M.A., Anderson B.D., Gray G.C. Environmental and aerosolized severe acute respiratory syndrome coronavirus 2 among hospitalized coronavirus disease 2019 patients. J. Infect. Dis. 2020;222:1798–1806. doi: 10.1093/infdis/jiaa575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biryukov J., Boydston J.A., Dunning R.A., Yeager J.J., Wood S., Reese A.L., Ferris A., Miller D., Weaver W., Zeitouni N.E., Phillips A., Freeburger D., Hooper I., Ratnesar-Shumate S., Yolitz J., Krause M., Williams G., Dawson D.G., Herzog A., Dabisch P., Wahl V., Hevey M.C., Altamura L.A. Increasing temperature and relative humidity accelerates inactivation of SARS-CoV-2 on surfaces. mSphere. 2020;5(4) doi: 10.1128/mSphere.00441-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J., Xing F., Liu J., Yip C.C., Poon R.W., Tsoi H.W., Lo S.K., Chan K.H., Poon V.K., Chan W.M., Ip J.D., Cai J.P., Cheng V.C., Chen H., Hui C.K., Yuen K.Y. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D., Xu H., Rebaza A., Sharma L., Dela Cruz C.S. Protecting health-care workers from subclinical coronavirus infection. Lancet Respir. Med. 2020;8:e13. doi: 10.1016/S2213-2600(20)30066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng V.C.C., Wong S.C., Chen J.H.K., Yip C.C.Y., Chuang V.W.M., Tsang O.T.Y., Sridhar S., Chan J.F.W., Ho P.L., Yuen K.Y. Escalating infection control response to the rapidly evolving epidemiology of the coronavirus disease 2019 (COVID-19) due to SARS-CoV-2 in Hong Kong. Infect. Control Hosp. Epidemiol. 2020;41:1–24. doi: 10.1017/ice.2020.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia P.Y., Coleman K.K., Tan Y.K., Ong S.W.X., Gum M., Lau S.K., Lim X.F., Lim A.S., Sutjipto S., Lee P.H., Son T.T., Young B.E., Milton D.K., Gray G.C., Schuster S., Barkham T., De P.P., Vasoo S., Chan M., Ang B.S.P., Tan B.H., Leo Y.S., Ng O.T., Wong M.S.Y., Marimuthu K. Singapore 2019 Novel Coronavirus Outbreak Research Team. Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nat. Commun. 2020 May 29;11(1):2800. doi: 10.1038/s41467-020-16670-2. PMID: 32472043; PMCID: PMC7260225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020;104:246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.H., Chang S.Y., Sung M., Park J.H., Bin Kim H., Lee H., Choi J.P., Choi W.S., Min J.Y. Extensive viable middle east respiratory syndrome (MERS) coronavirus contamination in air and surrounding environment in MERS isolation wards. Clin. Infect. Dis. 2016;63:363–369. doi: 10.1093/cid/ciw239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim U.J., Lee S.Y., Lee J.Y., Lee A., Kim S.E., Choi O.J., Lee J.S., Kee S.J., Jang H.C. Air and environmental contamination caused by COVID-19 patients: a multi-center study. J. Kor. Med. Sci. 2020 Sep 21;35(37):e332. doi: 10.3346/jkms.2020.35.e332. PMID: 32959546; PMCID: PMC7505729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Ning Z., Chen Y., Guo M., Liu Y., Gali N.K., Sun L., Duan Y., Cai J., Westerdahl D., Liu X., Xu K., Ho K.F., Kan H., Fu Q., Lan K. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582:557–560. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- Morawska L., Cao J. Airborne transmission of SARS-CoV-2: the world should face the reality. Environ. Int. 2020;139:105730. doi: 10.1016/j.envint.2020.105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y., Marimuthu K. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020;323:1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otter J.A., Donskey C., Yezli S., Douthwaite S., Goldenberg S.D., Weber D.J. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J. Hosp. Infect. 2016;92:235–250. doi: 10.1016/j.jhin.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzini K., Castrica M., Menchetti L., Maggi L., Negroni L., Orfeo N.V., Pizzoccheri A., Stocco M., Muttini S., Balzaretti C.M. SARS-CoV-2 RNA detection in the air and on surfaces in the COVID-19 ward of a hospital in Milan, Italy. Sci. Total Environ. 2020 Nov 10;742:140540. doi: 10.1016/j.scitotenv.2020.140540. Epub 2020 Jun 26. PMID: 32619843; PMCID: PMC7319646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riou J., Althaus C.L. Pattern of early human-to-human transmission of Wuhan 2019 novel coronavirus (2019-nCoV), December 2019 to January 2020. Euro Surveill. 2020 Feb;25(7) doi: 10.2807/1560-7917.ES.2020.25.4.2000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robotto A., Civra A., Quaglino P., Polato D., Brizio E., Lembo D. SARS-CoV-2 airborne transmission: a validated sampling and analytical method. Environ. Res. 2021;200:111783. doi: 10.1016/j.envres.2021.111783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D., Holbrook M., Gamble A., Williamson B., Tamin A., Harcourt J., Thornburg N., Gerber S., Lloyd-Smith J., de Wit E., Munster V. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Feng H., Zhang S., Ni Z., Ni L., Chen Y., Zhuo L., Zhong Z., Qu T. SARS-CoV-2 RNA detection of hospital isolation wards hygiene monitoring during the Coronavirus Disease 2019 outbreak in a Chinese hospital. Int. J. Infect. Dis. 2020 May;94:103–106. doi: 10.1016/j.ijid.2020.04.024. Epub 2020 Apr 18. PMID: 32311449; PMCID: PMC7165090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . World Health Organization; Geneva: 2014. Infection Prevention and Control of Epidemic- and Pandemic-Prone Acute Respiratory Infections in Health Care. [PubMed] [Google Scholar]
- WHO . 2020. Infection Prevention and Control during Health Care when Novel Coronavirus (nCoV) Infection Is Suspected: Interim Guidance.https://www.who.int/publications-detail/infection-prevention-and-control-during-health-care-when-novel-coronavirus-(ncov)-infection-is-suspected-20200125 25 Jan 2020. [Google Scholar]
- Zhao Q., Guo Y., Ye T., Gasparrini A., Tong S., Overcenco A., Urban A., Schneider A., Entezari A., Vicedo-Cabrera A.M., Zanobetti A., Analitis A., Zeka A., Tobias A., Nunes B., Alahmad B., Armstrong B., Forsberg B., Pan S.C., Íñiguez C., Ameling C., De la Cruz Valencia C., Åström C., Houthuijs D., Dung D.V., Royé D., Indermitte E., Lavigne E., Mayvaneh F., Acquaotta F., de'Donato F., Di Ruscio F., Sera F., Carrasco-Escobar G., Kan H., Orru H., Kim H., Holobaca I.H., Kyselý J., Madureira J., Schwartz J., Jaakkola J.J.K., Katsouyanni K., Hurtado Diaz M., Ragettli M.S., Hashizume M., Pascal M., de Sousa Zanotti Stagliorio Coélho M., Valdés Ortega N., Ryti N., Scovronick N., Michelozzi P., Matus Correa P., Goodman P., Nascimento Saldiva P.H., Abrutzky R., Osorio S., Rao S., Fratianni S., Dang T.N., Colistro V., Huber V., Lee W., Seposo X., Honda Y., Guo Y.L., Bell M.L., Li S. Global, regional, and national burden of mortality associated with non-optimal ambient temperatures from 2000 to 2019: a three-stage modelling study. Lancet Planet Health. 2021 Jul;5(7):e415–e425. doi: 10.1016/S2542-5196(21)00081-4. PMID: 34245712. [DOI] [PubMed] [Google Scholar]