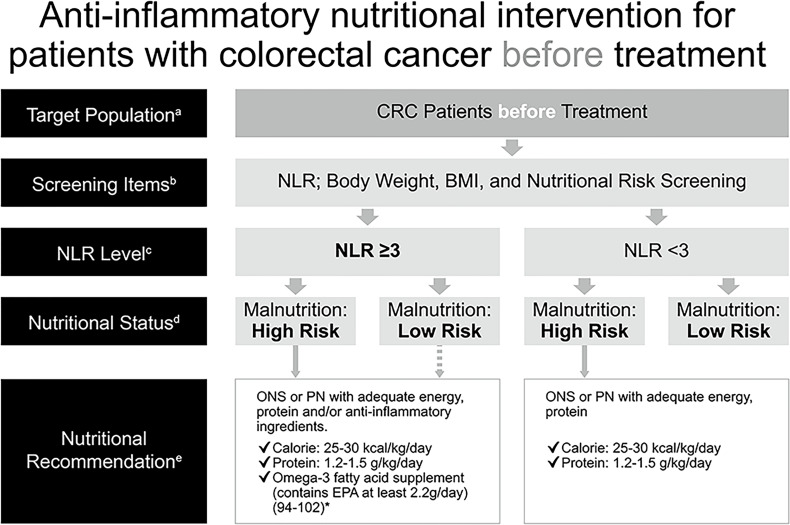
Figure 2.
Anti-inflammatory nutritional intervention for patients with colorectal cancer before treatment. *(References 94–102) aCRC patients before treatment (cancer-related drug treatment, radiotherapy and surgical treatment). bCommonly used clinical nutrition screening/evaluation tools in Taiwan, which include PG-SGA, NRS 2002, MUST, MST. cThe NLR test is used to assess the risk of inflammation. dInterpretation based on the evaluation/screening tools used in the nutrition evaluation clinical practice routines of each hospital. eBased on the recommendations of ESPEN 2017, ASPEN, and the Academy of Nutrition and Dietetics, for cancer, diseases, and treatments that may cause inflammation, insufficient calorie intake, fatigue and low physical activity, omega-3 fatty acids (such as fish oil, EPA) should be consumed for their anti-inflammatory effects, in addition to ONS which provides additional calories and protein. Nutritional intervention can be performed if the patient has a high risk of malnutrition. There is no need to distinguish between precachexia and cachexia–directly start supplementing calories, protein, and omega-3 fatty acids for nutritional intervention, according to clinical guidelines, without further screening. The dotted line: If the patient is not at high risk of malnutrition but has an NLR ≥3, the follow-up decision depends on the physician, and the physician (or a supervisor) will then call the nutritionist. ASPEN, American Society for Parenteral and Enteral Nutrition; BMI, body mass index; CRC, colorectal cancer; EPA, eicosapentaenoic acid; ESPEN, European Society for Parenteral and Enteral Nutrition; MST, Malnutrition Screening Tool; MUST, Malnutrition Universal Screening Tool; NG, nasogastric; NLR, neutrophil-to-lymphocyte ratio; NRS, Nutritional Risk Screening; ONS, oral nutritional supplement; PG-SGA, Patient-Generated Subjective Global Assessment; PN, parenteral nutrition.