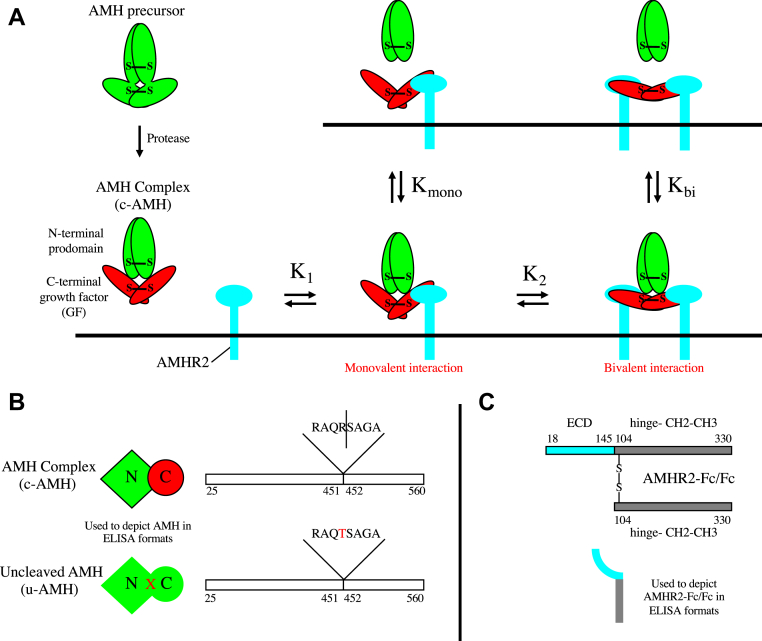
Figure 1.
Interaction of AMH with its type II receptor AMHR2.A, the AMH precursor undergoes an obligatory cleavage resulting in the noncovalent AMH complex that can bind to AMHR2, the type II receptor. After binding, the prodomain is released, but it has not been determined whether this is the result of a monovalent interaction or a bivalent interaction with AMHR2 or either interaction. In the current study, values for Kmono and Kbi have been derived showing that prodomain release occurs only after a bivalent interaction, which induces a change in the conformation of the GF. Both the GF and prodomain contain intermolecular disulfide bonds. B, reagents used for experiments in the current study. c-AMH was produced in Chinese hamster ovary cells as mostly precursor and converted to completely cleaved noncovalent complex by treatment with plasmin. u-AMH contains an R451T mutation at the cleavage sites between the N-terminal prodomain and the C-terminal GF domain. The numbering corresponds to amino acid residues in UniProtKB accession number P03971 (human AMH). C, AMHR2-Fc/Fc was generated by coexpressing a complementary DNA encoding an AMHR2-Fc fusion protein together with a complementary DNA encoding the Fc portion of IgG1 in human embryonic kidney 293 cells. The numbering corresponds to amino acid residues in UniProtKB accession numbers Q16671 (human AMHR2) and P01857 (human 1g γ-1 chain constant region). The representations of c-AMH and u-AMH (shown in B) and AMHR2-Fc/Fc (shown in C) are used to depict these molecules in ELISA formats. AMH, anti-Müllerian hormone; AMHR2, anti-Müllerian hormone receptor type-2; c-AMH, cleaved AMH; GF, growth factor; u-AMH, uncleaved AMH.