Abstract
The purpose of this study was to determine whether endurance (E) or endurance + resistance (ER) training affects C-reactive protein (CRP) and if these changes are related to alterations in fitness and (or) body composition in young females. Thirty-eight females (aged 18–24 years) were assigned to 1 of 3 groups: (1) E, (2) ER or (3) active control (AC). The E and ER groups completed 15 weeks of marathon training. The ER group performed additional resistance training and the AC group maintained their usual exercise routine. Primary outcomes were measured pre- and post-training and included anthropometric indices, dual-energy x-ray absorptiometry, plasma CRP, time to complete 1.5 miles (in minutes), and upper and lower body strength tests (i.e., 8 repetition max on bench and leg press (ER group only)). There were no differences in any variable among the groups at baseline. After training, the E group decreased time to complete 1.5 miles (p < 0.05). The AC group decreased percent and absolute body fat while the E group decreased percent body fat, absolute body fat, and android and gynoid body fat (p < 0.05). The ER group significantly improved strength (p < 0.001) and reduced plasma CRP from 2.0 ± 1.1 to 0.8 ± 0.3 mg·L–1 (p = 0.03). No significant associations were observed between CRP and measures of body composition or aerobic capacity. Combined endurance and resistance training may be an effective modality for reducing plasma CRP in young adult females independent of changes in aerobic capacity or body composition.
Keywords: inflammation, women, strength, aerobic, endurance, female
Introduction
Cardiovascular disease (CVD) accounts for nearly 40% of deaths annually in urbanized western countries (Albert and Ridker 2006). The identification and reduction of traditional risk factors such as smoking, sedentary lifestyle, high blood pressure and (or) cholesterol, obesity, and unrelieved stress have helped individuals slow the development and progression of the disease (Rutten et al. 2010). Despite these advances, cardiovascular events continue to occur in otherwise healthy individuals who do not exhibit these conditions. Furthermore, CVD is becoming more prevalent earlier in life (Cherian et al. 2009; Magkos et al. 2006). Current endeavors have turned to identifying and studying additional risk factors with a strong ability to predict CVD, namely lipoprotein, fibrinogen, homocysteine, and C-reactive protein (CRP) in both young and older populations (Hackam and Anand 2003).
Although low-density lipoprotein (LDL) is the main cholesterol-carrying protein known to play a role in CVD development and a well-established clinical marker of CVD (Jae et al. 2006), CRP has gained recognition as a significant biological marker of CVD and has been implicated as a stronger indicator of disease risk (Wilund 2007). CRP is an acute phase inflammatory protein released from the liver and adipose tissue that has been shown to bind to oxidized LDL. Elevated circulating CRP is linked to an increased occurrence of myocardial infarction, stroke, cardiovascular events, and peripheral arterial disease (Kaptoge et al. 2010; Rifai 2005). Elevated CRP is considered to be any level above 3 mg·L–1. Other risk categories for CVD can also be defined by circulating CRP levels with average risk between 1–3 mg·L–1 and low risk below 1 mg·L–1 (Pearson et al. 2003).
Regular physical activity is known to decrease the risk of CVD; however, the mechanism driving this reduction re-mains unclear (Kaptoge et al. 2010). One viable explanation for the beneficial effects of physical activity on lowering CVD risk involves reducing chronic inflammation (Wilund 2007). Although epidemiologic studies support an inverse relationship between CRP levels and regular physical activity levels (Church et al. 2002; Stewart et al. 2010), the merit of exercise interventions in decreasing CRP remains controversial and may be dependent on the intensity and modality of the exercise intervention as well as the study population. The majority of large aerobic exercise intervention studies show no change in CRP and this finding has become more consistent as researchers have been able to recruit a wide variety of subjects into longer, supervised intervention studies (Campbell et al. 2008; Church et al. 2010; Marcell et al. 2005; Rauramaa et al. 2004; Stewart et al. 2010). Despite the lack of change in CRP observed in response to aerobic training, a growing body of evidence supports the potential for resistance training (RT) to effectively lower CRP (Donges et al. 2010; Olson et al. 2007; Stewart et al. 2007). However, with no consensus on the topic and a lack of research specifically targeting an answer, there is a need for more insight about the potential for RT combined with aerobic training to positively affect CRP and CVD risk.
The purpose of this study was to determine whether endurance or endurance combined with RT influences circulating CRP levels in a young, healthy female population. We hypothesized that the combination of endurance and RT would have a more pronounced positive effect on CRP levels than endurance training alone. Since CVD is becoming more prevalent at younger ages (Cherian et al. 2009; Magkos et al. 2006), this study not only offers information about endurance and RT for health and fitness adaptations as well as provides support to establish the efficacy of RT in lowering CRP, but it also allows for a much needed evaluation of CRP in a younger population.
Materials and methods
Study subjects
Young adult females (aged 18–24 years) were recruited from Louisiana State University (Baton Rouge, La., USA). All participants were healthy, not pregnant, and able to complete a vigorous exercise training plan. All participants completed an informed consent and medical history and received physician approval prior to beginning the training program. This project was approved by the Louisiana State University Institutional Review Board.
Study design
Participants were allowed to choose inclusion into 1 of 2 groups: (1) endurance training (E; N = 11) or (2) combined endurance and RT (ER; N = 7). Subsequent inclusion of participants in the E and ER groups was dependent on the participants not having participated in a half marathon or longer race distance. In addition, E and ER participants must have been able to complete a 1.5-mile run before the intervention period and enrollment into the study. The E group completed a periodized, progressive 15-week marathon training regimen (Table A1) that included 3 runs each week with 1 supervised long run and cross-training on the weekend days. The duration of the aerobic workouts varied depending upon each individual’s running pace as the runs were distance-based. The ER group performed a 14-week periodized RT regimen (Table A2) that was designed for runners and reviewed by a certified strength and conditioning specialist, in addition to an altered, periodized, progressive 15-week marathon training plan (Table A1) to account for the increase in workload. The alteration involved ER group participants running an average of 16% fewer miles per week compared with the E program. Subjects in the ER group completed 2 supervised, non-consecutive RT sessions each week. The periodized RT plan consisted of a preparatory phase (3 weeks), training phase (7 weeks), peak phase (1 week), and taper phase (3 weeks)(Table A2). The duration of the RT sessions varied depending upon each individual’s workout pace, but rarely ex-ceeded1 h. All training was documented weekly and monitored to ensure adherence and progress. Active individuals were recruited into the active control group (AC; N = 20). Enrollment into the AC group required participation in a consistent workout plan with at least 3 days of an activity for at least 1 year prior to the start of the study. The AC group was instructed to maintain their normal daily activities.
Measures
All pre- and postmeasurements were conducted at the beginning and end of the intervention period, respectively. Post-measurements were taken during the last week of the taper period and before the E and ER group participants competed in a marathon event.
Cardiorespiratory testing
Aerobic capacity was estimated by using a common field test equation derived to estimate maximal oxygen consumption from 1.5-mile run time as previously described (American College of Sports Medicine 2009). The 1.5-mile run was performed on an indoor track.
Strength fitness testing
For those subjects in the ER group, an 8-repetition maximum (8RM) on bench and leg presses was used to determine potential gains in strength associated with the RT plan. All 8RM lifts were supervised by a strength and conditioning expert.
Anthropometry
All anthropometric measures were conducted by the same technician pre- and post-intervention to ensure consistency. Height was measured using a Shorr stadiometer (Shorr Productions, Olney, Md., USA) and weight was recorded using a digital scale (SECA 880), calibrated daily with two 5-kg weights. Body mass index (BMI) values were calculated as weight in kilograms divided by height in meters squared (kg·m–2). Waist and hip circumferences were measured with a Gulick tension tape and were used to calculate the waist/hip ratio (WHR) as previously described (American College of Sports Medicine 2009).
Dual-energy x-ray absorptiometry (DXA)
Whole-body DXA scans were performed using a full-size Prodigy Pro apparatus (GE Lunar Corporation, Madison, Wis., USA) and processed with Encore 2004 software (version 8.10.027). Measurements of absolute and relative (%) fat mass (FM), bone-free lean-tissue mass (LTM), and bone mineral content (BMC) were collected. Primary outcome measures included change in total percent body fat (%BF), android %BF, and gynoid %BF. All scans were conducted by a trained technician and spot checks for accuracy were verified by a certified densitometry technologist. Participants were asked to refrain from exercise 24 h prior to measurements.
Blood collection–analysis
Fasting, resting blood samples (20 mL) were taken pre-and post- intervention period. Participants were asked to refrain from exercise and eat the same diet 24 h prior to blood collection to limit potential diet-induced alterations. Subjects reported to the Exercise Biochemistry Laboratory at Louisiana State University between 0500–0900 hours where a registered nurse collected blood into EDTA vacutainers after an overnight fast. Blood samples were immediately centrifuged at 4 °C (10 min, 1000 r·min–1 (1200g)) and plasma was aspirated, aliquoted, and stored at –80 °C until analysis. Immediately post-intervention, CRP was determined in samples from all time points by commercially available enzyme-linked immunosorbent assay kits (Alpco C-Reactive Protein (hsCRP) EIA, Salem, N.H., USA) with a BioTek microplate reader (BioTek Instruments, Model MQX200, Winooski, Vt., USA). Intra- and inter-assay coefficients of variation were less than 2%.
Statistical analysis
Data were analyzed using SAS (version 9.1.3; SAS Institute Inc., Cary, N.C., USA). Descriptive baseline statistics were computed by treatment groups and compared using Student’s t tests. Mixed model analysis of variance tests using a 2-way repeated-measures ANOVA (SAS PROC MIXED) with treatment and time as fixed effects and subjects as the random effect were computed to test for significant differences in weight, body mass index (BMI), WHR, 1.5-mile run test, strength, CRP, and DXA variables. CRP values were log-transformed prior to analysis. All interactions were considered in the models. Post hoc tests using Dunnett’s method were used to adjust the significance level of the least square means for multiple comparisons. Pearson stepwise correlations analysis was used to determine relationships between all variables. Significance was set at an α level of p < 0.05.
Results
Performance outcomes
All subjects in the E and ER groups completed the training program and individual session compliance was at least 92%. There were no significant differences between groups at baseline for the 1.5-mile run time to completion. At the post-intervention time point, the E group experienced improvement (p < 0.05) in the 1.5-mile run field test while the AC and ER groups did not change significantly. For the ER group only, 8RM was assessed to determine strength gains due to the addition of RT. The average bench press and leg press 8RM increased 16.1% and 87.3%, respectively (p = 0.0002; p < 0.0001) (Table 1).
Table 1.
Participant characteristics.
| AC group | E group | ER group | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Variable | Pre | Post | Pre | Post | Pre | Post |
| Age(y) | 21.3±0.3 | 21.4±0.2 | 21.4±0.3 | |||
| Height (cm) | 164.8±0.9 | 166.1 ± 1.3 | 166.1±1.3 | |||
| Weight (kg) | 135.3±4.7 | 133.4±4.5 | 140.3 ±4.4 | 139.0±4.6 | 137.5 ±5.9 | 138.6±5.1 |
| BMI | 22.5±0.7 | 22.3 ±0.7 | 23.0±0.6 | 23.2±0.8 | 22.7±0.5 | 22.8 ±0.5 |
| WHR | 0.75±0.01 | 0.73 ±0.03 | 0.72±0.04 | 0.72±0.04 | 0.76±0.02 | 0.78±0.02 |
| 1.5 mile time (min) | 14.4±0.3 | 13.8±0.4 | 14.1 ±0.3 | 11.8±0.3* | 13.1±0.4 | 11.8 ±0.3 |
| 8RM BP (kg) | 30.4±2.2 | 35.3±2.6† | ||||
| 8RM LP (kg) | 48.1 ±5.8 | 90.1 ±7.2† | ||||
Note: Data are presented as least-square means ± SE. AC, control; E, endurance, ER, endurance + resistance training; BMI, body mass index; WHR, waist/hip ratio; 8RM, 8-repetition maximum; BP, bench press; LP, leg press.
p < 0.05 for mean difference between pre- and post-test.
p < 0.001 for mean difference between pre- and post-test.
Body Composition
The mean anthropometric measurements taken at pre- and post-intervention for all participants are shown in Table 1. There were no differences in height, weight, BMI, WHR, or BMC by time or treatment group.
Significant time by treatment differences were observed in the E group and AC group for absolute FM and %BF (Ta-ble 2). In the E group, absolute fat mass decreased 6.9%(p < 0.05) and %BF decreased 5.1% (p < 0.05). In the AC group, absolute fat mass decreased 6.0% (p < 0.05) and %BF decreased 4.3% (p < 0.05). A significant reduction in both android %BF (7.7%; p < 0.05) and gynoid %BF (3.8%, p < 0.05) was observed in the E group. At baseline, there was no difference in LTM among the 3 groups. After the intervention, the ER group gained (2.9%) LTM; how-ever, no significant changes in LTM were observed among the groups (Table 2). There were no significant correlations between measures of body composition, CRP, or 1.5-mile time at pre- or post-intervention time points.
Table 2.
Body composition measures.
| AC group | E group | ER group | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| DXA variable | Pre | Post | Pre | Post | Pre | Post |
| FM (kg) | 18.44±1.22 | 17.34±1.22* | 22.12±1.64 | 20.59±1.64* | 18.64±1.76 | 17.29±1.61 |
| LTM (kg) | 39.74±0.88 | 39.94±0.94 | 39.19± 1.35 | 39.54±1.42 | 41 55±1.34 | 42.75±1.02 |
| BMC (kg) | 2.456±0.080 | 2.481 ±0.076 | 2.556±0.011 | 2.560±0.011 | 2.524±0.016 | 2.521 ±0.017 |
| Total %BF | 29.73±1.23 | 28.46±1.23* | 34.47±1.66 | 32.71±1.66* | 29.03±2.08 | 27.34±2.08 |
| Android %BF | 33.30±2.20 | 32.20±2.20 | 37.66±2.70 | 34.75±2.70* | 31.50±3.50 | 29.60±3.00 |
| Gynoid %BF | 40.30±1.10 | 40.00±1.20 | 44.75±1.33 | 43.07±1.33* | 39.70±1.40 | 37.70±1.20 |
Note: Data are presented as least-square means ± SE. AC, control; E, endurance; ER, endurance + resistance training; DXA, dual energy x-ray absorptiomeLry; FM, fat mass; LTM, bone-free lean-tissue mass; BMC, bone mineral content; %BF, percent body fat.
p < 0.05 for mean difference between pre- and post-test.
Plasma CRP levels
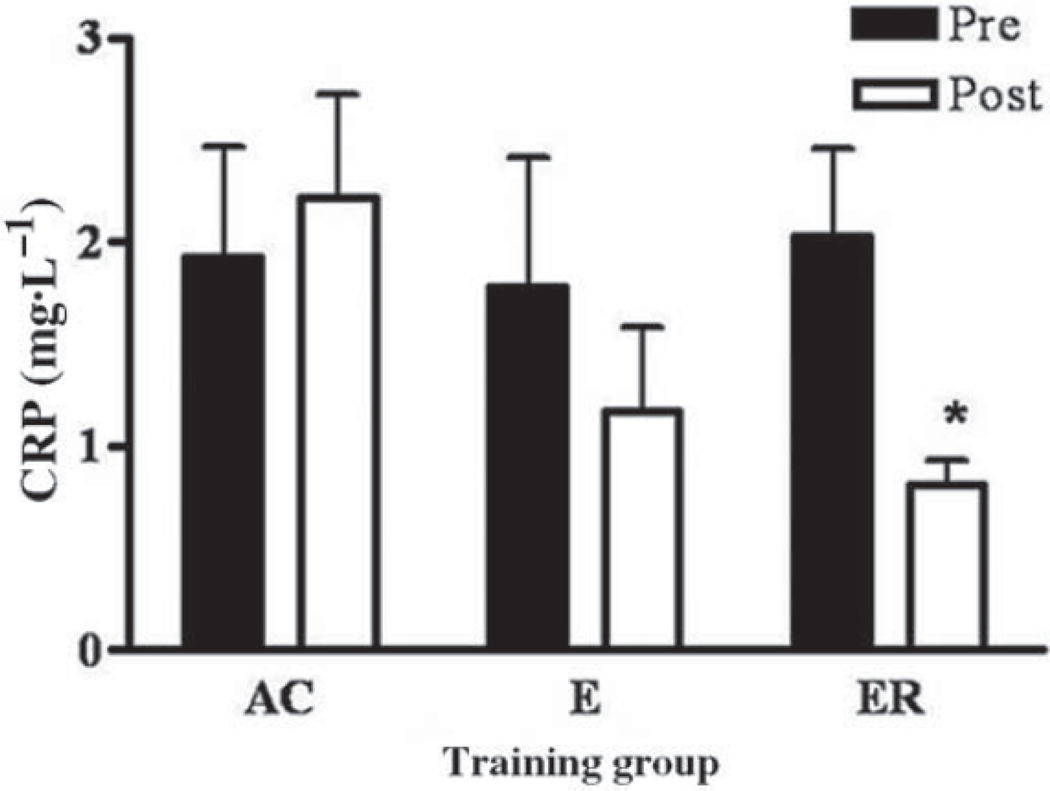
There were no significant differences in CRP levels among the 3 groups at baseline. After participating in the intervention, only the ER group significantly decreased (60.0%) plasma CRP (2.0 ± 1.1 to 0.8 ± 0.3 mg·L–1 (mean ± SD); p = 0.03). The E group decreased (34.3%) plasma CRP from 1.6 ± 2.0 to 1.1 ± 1.8 mg·L–1 while the AC group in-creased (15.2%) plasma CRP from 1.9 ± 2.5 to 2.2 ± 2.1 mg·L–1 (Fig. 1). Pre- or post-CRP was not correlated with any of our outcome variables.
Fig. 1.
C -reactive protein (CRP) expressed in milligrams per litre (pre and post) for the active control (AC) group n = 20, endurance (E) group n =11, and endurance + resistance (ER) group n = 7. *, ER post-CRP value is significantly lower than ER pre-CRP value (p = 0.03). Values are means ± SE.
Discussion
The results of this study suggest that RT combined with endurance training plays a significant role in lowering CRP independent of changes in cardiovascular fitness or measures of adiposity in young women. The addition of RT allowed the ER group to move from the average CVD risk category as defined by CRP (1–3 mg·L–1) to the low risk category (<1 mg·L–1). Our findings are supported by other studies that have shown that the addition of resistance training may be more effective than aerobic training alone in decreasing CRP levels (Donges et al. 2010; Stewart et al. 2007). The exploration of the efficacy of RT to reduce CVD risk, assessed by CRP, in younger individuals is particularly important given that CVD is now becoming common in individuals at earlier ages (Arsenault et al. 2009) and provides support for the incorporation of supervised, progressive strength training programs in college-aged females.
The mechanism whereby exercise training alters CRP re-mains unknown, but decreases in CRP levels have been correlated with changes in cardiorespiratory fitness (Jae et al. 2006), BMI and adiposity (Arsenault et al. 2009; Church et al. 2010; Nicklas et al. 2008). In the present study, we observed an improvement in cardiovascular fitness despite a lack of change in CRP in the E group, suggesting that im-proved cardiorespiratory fitness is not essential in eliciting beneficial changes in CRP. Such results are in contrast to existing literature that showed a relationship between improvements in CRP and aerobic fitness (Jae et al. 2006). In a similar study, 9 months of marathon training resulted in improvements in aerobic capacity and circulating CRP levels (Mattusch et al. 2000). The current study, however, included participants undergoing marathon training for 15 weeks, less than half the time used for marathon training in the previous study (Mattusch et al. 2000). It is possible that greater improvements in CRP in the E group would have been observed in response to a longer training period.
After the intervention period, we observed a reduction in FM and %BF in both the AC and E groups. While the reduction in FM in the AC group was unexpected, it is important to note that those in the AC group were peers of those participating in the marathon and RT programs. The AC group did not improve 1.5-mile time performance or decrease CRP, but it is possible that they began to adopt healthier habits that led to decreases in FM during the study. Even though larger, controlled clinical trials support a relationship between BMI, waist circumference, adiposity, and CRP (Arsenault et al. 2009; Church et al. 2010; Nicklas et al. 2008), we found that changes in %BF and FM were not related to decreases in CRP. In addition, anthropometric measurements, including BMI and WHR, were also not related to changes in CRP. As a result, the relationship between these variables and CRP re-mains unclear.
While the AC group exhibited overall decreases in FM, only the E group experienced a reduction in android and gynoid %BF after the intervention period. The android region is considered to be the more unhealthy area for fat to accumulate, and android fat content is positively related to CRP (Rutten et al. 2010). Although there was no correlation be-tween CRP and android body fat, it is essential to understand that the measure of android fat taken in this study is different from the measure of visceral fat observed in other studies (Kim et al. 2008). Changes in CRP also did not correlate with changes in absolute or relative FM. It is interesting to note, however, that while CRP was not related to changes in LTM, CRP decreased significantly in only the ER group, which was the only group to experience an improvement in LTM. This trend suggests a potential link between reductions in CRP and increases in lean muscle mass.
There were several study limitations that deserve mention. Despite the significant results with respect to changes in CRP, there were a small number of participants in the ER group. Post hoc analysis indicated an observed power (p = 0.05) of 0.53 to detect differences in CRP in the ER group. However, a study involving marathon training (Mattusch et al. 2000) produced noteworthy results in a similar sample size of 12 runners and 10 controls. A second limitation of the present study is the lack of 8RM data in E and AC groups. It is well-documented that endurance training alone produces little to no gain in strength (Dudley and Djamil 1985; Hickson 1980). Thus, we felt that it was unnecessary to assess pre- and post-8RM in E and AC groups. In retrospect, 8RM assessment in all groups would have given us a better understanding of how RT leads to changes in CRP levels. Another limitation in the present study is the lack of change in aerobic fitness in the ER group as measured by the 1.5-mile timed run. This may be explained by the observed higher aerobic fitness level in the ER group at base-line. The ER group had a 7.6% faster 1.5-mile time than the E group at baseline, although this difference was insignificant. As a result, the ER group had less room for improvement than the E group. In fact, the E group improved by 16.3% in their 1.5-mile run time while the ER group experienced a 10% improvement in 1.5-mile time. A final limitation was that all participants were young and healthy adult females. The majority of CRP-related research has been among the middle-aged and diseased populations that often allow for more pronounced changes in CRP. Due to the fact that CRP level is highly predictive of relative risk for future cardiovascular events, this limitation can also be considered a strength because it allows for more available information about the ability of exercise to alter CRP levels in young people (Albert and Ridker 2006; Ridker and Silvertown 2008). Further-more, there are often many concerns with use of female participants due to various physiological changes that occur throughout the menstrual cycle. Evidence from 2 studies sup-ports no significant change in CRP during the different stages of the menstrual cycle (Capobianco et al. 2010; Wunder et al. 2006).
In conclusion, combined endurance and resistance training resulted in significant decreases in CRP in young females. This reduction was independent of changes in fitness and body composition. Given the findings reported by this study and others, there is a growing body of evidence that supports the novel action of strength training in reducing CRP.
Supplementary Material
Acknowledgements
The authors thank the participants for their hard work and dedication to the study.
Contributor Information
Laura A. Daray, Department of Kinesiology, Louisiana State University, Baton Rouge, LA 70803, USA.
Tara M. Henagan, Department of Kinesiology, Louisiana State University, Baton Rouge, LA 70803, USA Antioxidant and Gene Regulation Laboratory, Pennington Biomedical Research Center, Baton Rouge, LA 70808, USA..
Michael Zanovec, School of Human Ecology, Louisiana State University Agricultural Center, Baton Rouge, LA 70803, USA..
Conrad P. Earnest, Exercise Biology and Testing Core, Pennington Biomedical Research Center, Baton Rouge, LA 70808, USA.
Lisa G. Johnson, Department of Kinesiology, Louisiana State University, Baton Rouge, LA 70803, USA.
Jason Winchester, Department of Kinesiology, Leisure, and Sport Sciences, East Tennessee State University, P.O. Box 70654, Johnson City, TN 37614, USA..
Georgianna Tuuri, School of Human Ecology, Louisiana State University Agricultural Center, Baton Rouge, LA 70803, USA..
Laura K. Stewart, Department of Kinesiology, Louisiana State University, Baton Rouge, LA 70803, USA.
References
- Albert MA, and Ridker PM 2006. C-reactive protein as a risk predictor: do race/ethnicity and gender make a difference? Circulation, 114(5): e67–e74. doi: 10.1161/CIRCULATIONAHA.106.613570. PMID:16880331. [DOI] [PubMed] [Google Scholar]
- American College of Sports Medicine. 2009. ACSM’s Guidelines for Exercise Testing and Prescription. Lippincott Williams & Wilkins. p.298. [Google Scholar]
- Arsenault BJ, Earnest CP, Despres JP, Blair SN, and Church TS 2009. Obesity, coffee consumption and CRP levels in postmenopausal overweight/obese women: importance of hormone replacement therapy use. Eur. J. Clin. Nutr. 63(12): 1419–1424. doi: 10.1038/ejcn.2009.112. PMID:19756031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KL, Campbell PT, Ulrich CM,Wener M,Alfano CM, Foster-Schubert K, et al. 2008. No reduction in C-reactive protein following a 12-month randomized controlled trial of exercise in men and women. Cancer Epidemiol. Biomarkers Prev. 17(7): 1714–1718. doi: 10.1158/1055-9965.EPI-08-0088. PMID:18628422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capobianco G, de Muro P, Cherchi GM, Formato M, Lepedda AJ, Cigliano A, et al. 2010. Plasma levels of C-reactive protein, leptin and glycosaminoglycans during spontaneous menstrual cycle: differences between ovulatory and anovulatory cycles. Arch. Gynecol. Obstet. 282(2): 207–213. doi: 10.1007/s00404-010-1432-2. PMID:20306065. [DOI] [PubMed] [Google Scholar]
- Cherian B, Meka N, Katragadda S, and Arora R. 2009. Therapeutic implications of diabetes in cardiovascular disease. Am. J. Ther. 16(6): e51–e59. doi: 10.1097/MJT.0b013e31815db924. PMID:19940606. [DOI] [PubMed] [Google Scholar]
- Church TS, Barlow CE, Earnest CP, Kampert JB, Priest EL, and Blair SN 2002. Associations between cardiorespiratory fitness and C-reactive protein in men. Arterioscler. Thromb. Vasc. Biol. 22(11): 1869–1876. doi: 10.1161/01.ATV.0000036611.77940.F8. PMID:12426218. [DOI] [PubMed] [Google Scholar]
- Church TS, Earnest CP, Thompson AM, Priest EL, Rodarte RQ, Saunders T, et al. 2010. Exercise without weight loss does not reduce C-reactive protein: the INFLAME study. Med. Sci. Sports Exerc. 42(4): 708–716. doi: 10.1249/MSS.0b013e3181c03a43. PMID:19952828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donges CE, Duffield R, and Drinkwater EJ 2010. Effects of resistance or aerobic exercise training on interleukin-6, C-reactive protein, and body composition. Med. Sci. Sports Exerc. 42(2): 304–313. doi: 10.1249/MSS.0b013e3181b117ca. PMID: 20083961. [DOI] [PubMed] [Google Scholar]
- Dudley GA, and Djamil R. 1985. Incompatibility of enduranceand strength-training modes of exercise. J. Appl. Physiol. 59(5): 1446–1451. PMID:4066574. [DOI] [PubMed] [Google Scholar]
- Hackam DG, and Anand SS 2003. Emerging risk factors for atherosclerotic vascular disease: a critical review of the evidence. JAMA, 290(7): 932–940. doi: 10.1001/jama.290.7.932. PMID: 12928471. [DOI] [PubMed] [Google Scholar]
- Hickson RC 1980. Interference of strength development by simultaneously training for strength and endurance. Eur. J. Appl. Physiol. Occup. Physiol. 45(2–3): 255–263. doi: 10.1007/BF00421333. PMID:7193134. [DOI] [PubMed] [Google Scholar]
- Jae SY, Fernhall B, Heffernan KS, Jeong M, Chun EM, Sung J, et al. 2006. Effects of lifestyle modifications on C-reactive protein: contribution of weight loss and improved aerobic capacity. Metabolism, 55(6): 825–831. doi: 10.1016/j.metabol.2006.02.010. PMID:16713444. [DOI] [PubMed] [Google Scholar]
- Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, and Danesh J; Emerging Risk Factors Collaboration. 2010. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet, 375(9709): 132–140. doi: 10.1016/S0140-6736(09)61717-7. PMID:20031199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Valentine RJ, Shin Y, and Gong K. 2008. Associations of visceral adiposity and exercise participation with C-reactive protein, insulin resistance, and endothelial dysfunction in Korean healthy adults. Metabolism, 57(9): 1181–1189. doi: 10.1016/j.metabol.2008.04.009. PMID:18702942. [DOI] [PubMed] [Google Scholar]
- Magkos F, Piperkou I, Manios Y, Papoutsakis C, Yiannakouris N, Cimponerio A, et al. 2006. Diet, blood lipid profile and physical activity patterns in primary school children from a semirural area of Greece. J. Hum. Nutr. Diet. 19(2): 101–112, quiz 113–116. doi: 10.1111/j.1365-277X.2006.00675.x. PMID: 16533372. [DOI] [PubMed] [Google Scholar]
- Marcell TJ, McAuley KA, Traustadottir T, and Reaven PD 2005. Exercise training is not associated with improved levels of C-reactive protein or adiponectin. Metabolism, 54(4): 533–541. doi: 10.1016/j.metabol.2004.11.008. PMID:15798963. [DOI] [PubMed] [Google Scholar]
- Mattusch F, Dufaux B, Heine O, Mertens I, and Rost R. 2000. Reduction of the plasma concentration of C-reactive protein following nine months of endurance training. Int. J. Sports Med. 21(1): 21–24. doi: 10.1055/s-2000-8852. PMID:10683094. [DOI] [PubMed] [Google Scholar]
- Nicklas BJ, Hsu FC, Brinkley TJ, Church T, Goodpaster BH, Kritchevsky SB, and Pahor M. 2008. Exercise training and plasma C-reactive protein and interleukin-6 in elderly people. J. Am. Geriatr. Soc. 56(11): 2045–2052. doi: 10.1111/j.1532-5415.2008.01994.x. PMID:19016938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson TP, Dengel DR, Leon AS, and Schmitz KH 2007. Changes in inflammatory biomarkers following one-year of moderate resistance training in overweight women. Int. J. Obes. (Lond.) 31(6): 996–1003. doi: 10.1038/sj.ijo.0803534. PMID: 17299382. [DOI] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO 3rd, Criqui M, et al. ; Centers for Disease Control and Prevention; American Heart Association. 2003. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation, 107(3): 499–511. doi: 10.1161/01.CIR.0000052939.59093.45. PMID:12551878. [DOI] [PubMed] [Google Scholar]
- Rauramaa R, Halonen P, Vaisanen SB, Lakka TA, Schmidt-Trucksass A, Berg A, et al. 2004. Effects of aerobic physical exercise on inflammation and atherosclerosis in men: the DNASCO Study: a six-year randomized, controlled trial. Ann. Intern. Med. 140(12): 1007–1014. PMID:15197018. [DOI] [PubMed] [Google Scholar]
- Ridker PM, and Silvertown JD 2008. Inflammation, C-reactive protein, and atherothrombosis. J. Periodontol. 79(Suppl. 8): 1544–1551. doi: 10.1902/jop.2008.080249. PMID:18673009. [DOI] [PubMed] [Google Scholar]
- Rifai N. 2005. High-sensitivity C-reactive protein: a useful marker for cardiovascular disease risk prediction and the metabolic syndrome. Clin. Chem. 51(3): 504–505. doi: 10.1373/clinchem.2004.044990. PMID:15738514. [DOI] [PubMed] [Google Scholar]
- Rutten EP, Breyer MK, Spruit MA, Hofstra T, van Melick PP, Schols AM, and Wouters EFM 2010. Abdominal fat mass contributes to the systemic inflammation in chronic obstructive pulmonary disease. Clin. Nutr. 29(6): 756–760. doi: 10.1016/j.clnu.2010.04.007. PMID:20566396. [DOI] [PubMed] [Google Scholar]
- Stewart LK, Flynn MG, Campbell WW, Craig BA, Robinson JP, Timmerman KL, et al. 2007. The influence of exercise training on inflammatory cytokines and C-reactive protein. Med. Sci. Sports Exerc. 39(10): 1714–1719. doi: 10.1249/mss.0b013e31811ece1c. PMID:17909397. [DOI] [PubMed] [Google Scholar]
- Stewart LK, Earnest CP, Blair SN, and Church TS 2010. Effects of different doses of physical activity on C-reactive protein among women. Med. Sci. Sports Exerc. 42(4): 701–707. PMID: 19952829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilund KR 2007. Is the anti-inflammatory effect of regular exercise responsible for reduced cardiovascular disease? Clin. Sci. (Lond.), 112(11): 543–555. doi: 10.1042/CS20060368. PMID:17459004. [DOI] [PubMed] [Google Scholar]
- Wunder DM, Yared M, Bersinger NA, Widmer D, Kretschmer R, and Birkhauser MH 2006. Serum leptin and C-reactive protein levels in the physiological spontaneous menstrual cycle in reproductive age women. Eur. J. Endocrinol. 155(1): 137–142. doi: 10.1530/eje.1.02178. PMID:16793960. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.