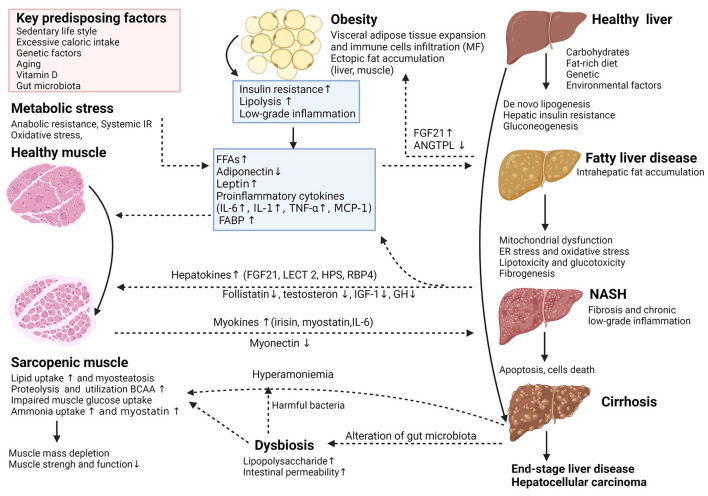
Figure 1.
(Created with BioRender.com). Key cellular and molecular mechanisms involved in the complex interplay between adipose tissue, sarcopenia, and the nonalcoholic fatty liver disease (NAFLD). The potential interaction between adipose tissue and skeletal muscle plays an essential role in the pathophysiological and natural course of NAFLD. Adipose tissue dysfunction is characterized by inflammation and adipokine disturbances, subsequent ectopic fat deposition and insulin resistance (IR). In insulin-resistant subjects, insulin fails to promote glycogen synthesis and favors adipose tissue lipolysis, redirecting substrate to “de novo” lipogenesis and accelerates proteolysis. These deregulations trigger further insults in hepatocytes through increased inflammation, lipotoxicity, mitochondrial dysfunction, oxidative and endoplasmic reticulum stress and anabolic resistance, which can all contribute to the progression of NAFLD. The release of multidirectional molecular signals comprising myokines and hepatokines regulates a range of systemic metabolic processes including skeletal muscle and hepatic IR, escalating dysfunction of the adipose-muscle-liver axis. Other mechanisms like dysbiosis related to changes in gut microbiota may have additional detrimental effects. Thus, dysregulation of the complex physiological relationship between skeletal muscle and the liver is reciprocally unfavorable and supports each other in a vicious circle, potentially playing a causative role in NAFLD incidence or progression. ANGPTL4, angiopoietin-like 4; BCAA, branched-chain amino acids; ER, endoplasmic reticulum; FABP, fatty acid-binding protein; FFA, free fatty acid; FGF21, fibroblast growth factor 21; GH, growth hormone; HPS, hepassocin; IGF-1, insulin-like growth factor 1; IL-6, interleukin-6; LECT2, leukocyte cell-derived chemotaxin-2; MCP-1, monocyte chemoattractant protein 1; MF, macrophages; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; RBP4, retinolbinding protein 4; TNF-α, tumor necrosis factor-alpha.