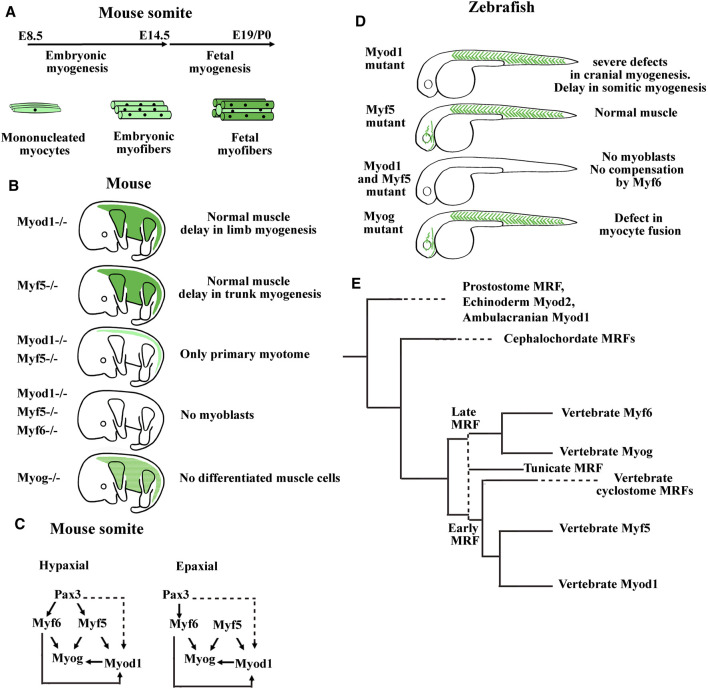
FIGURE 4.
Summary of myogenic regulatory factor (MRF) functions in mouse and zebrafish models. (A) Timing of myofibers formation during embryonic and fetal myogenesis in mice. (B) Summary of the main phenotypes of single KO mice for Myod1, Myf5, or Myogenin, double KO mice for Myod and Myf5, and triple KO mice for Myod1, Myf5, and Myf6. Myoblasts formation requires either Myod1 or Myf5. Myog is necessary for muscle differentiation and Myf6 can compensate the absence of Myod1 and Myf5 only during embryonic myogenesis. Myf6 KO mice did not show muscle development defects. Modified from Hernández-Hernández et al. (2017). (C) Genetic hierarchy during embryonic myogenesis in epaxial and hypaxial domain of the somite. Mice invalidated for the three genes Pax3, Myf5, and Myf6 show an absence of all skeletal muscles in the trunk indicating that these factors act upstream of Myod1. While the myogenesis in the hypaxial domain is Pax3 dependent, a program initiated by Myf5, Pax3 independent exists in the epaxial domain. Dashed lines indicate that the regulation of Myod1 expression by Pax3 is probably indirect through Pitx2 and Six transcription factors. Head mouse myogenesis is not presented here. For a more detailed analysis of MRF KO mice, see Bismuth and Relaix (2010), Comai et al. (2014), and Buckingham (2017). (D) Main phenotypes of zebrafish single mutants for Myod1, Myf5, or Myog and double mutant for Myod1 and Myf5. The zebrafish mutant for Myf6 did not show abnormal muscle development. In zebrafish, Myod1 is necessary for normal cranial muscle development whereas Myf6 does not compensate the absence of Myod1 and Myf5. (E) Summary of the MRF phylogeny in bilaterians. Aase-Remedios et al. (2020) propose that the four vertebrate genes coding for MRFs do not result from two rounds of whole genome duplication (2R WGD) of a single ancestral gene, that would have taken place between ancestral chordates and vertebrates. Instead, a cluster of two MRF genes generated by tandem duplication predates the 2R WGD. One gene of this cluster generates via 2R WGD and gene losses the early vertebrate MRFs (Myf5 and Myod1), and the other generates the late vertebrate MRFs (Myf6 and Myogenin). The first vertical dashed line indicates that tunicate MRFs could be the orthologs of either the early or the late ancestral MRF gene preceding the 2R WGD. The two MRF genes present in cyclostome species could be the orthologs of the early MRF gene. Cyclostomes would have diverged from gnathostomes after the first R WGD and before the second R WGD (Nakatani et al., 2021). Horizontal dashed lines indicate that the next duplication events in the branch are not shown here. Modified from Aase-Remedios et al. (2020).