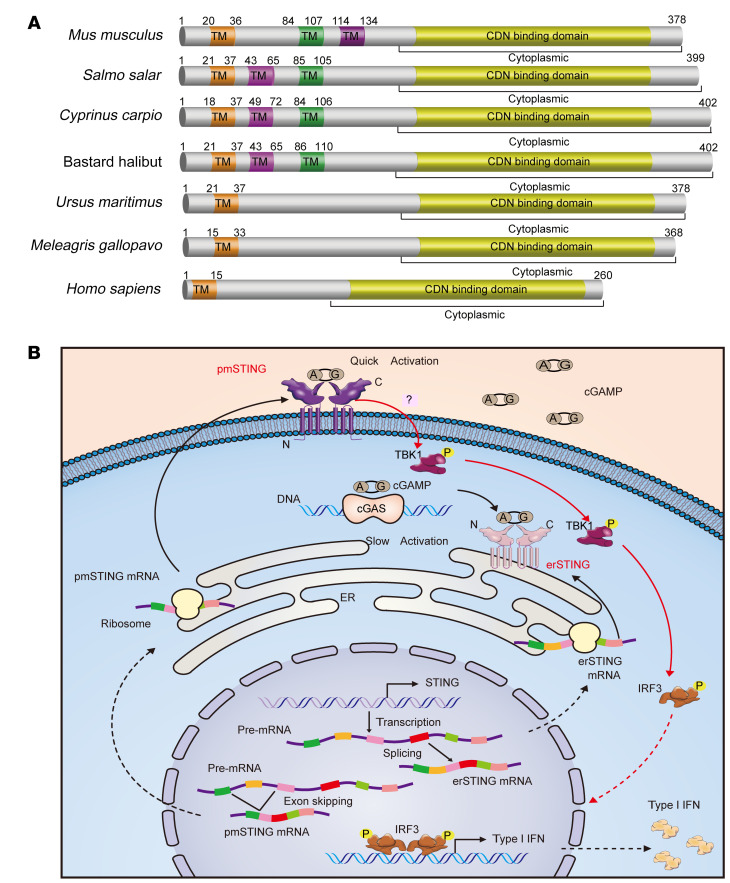
Figure 7. Schematic of the predicted pmSTING in various species and model of pmSTING sensing extracellular cGAMP and prompting IFN production.
(A) Multiple protein sequence alignment of STING proteins orthologous to the mouse pmSTING isoform. The STING proteins in different species shown here meet the following criteria: (a) possess an odd number of predicted TM domains; and (b) possess a C-terminal domain identical to that of the respective species’ canonical STING protein. (B) Model of the generation of 2 alternatively spliced STING isoforms and how they sense extracellular and intracellular cGAMP, respectively. Two STING transcripts generated by alternative splicing are translated in the cytoplasm and transported to the pmSTING and the endoplasmic reticulum (erSTING), respectively. Upon binding the intracellular cGAMP synthesized by cytosolic DNA–activated cGAS, erSTING undergoes homodimerization and translocates from endoplasmic reticulum to the perinuclear area, where it recruits and activates TBK1, which phosphorylates the transcription factor IRF3 and results in the translocation of IRF3 from the cytoplasm to the nucleus to induce the transcription of IFN and other immune cytokines. By contrast, the extracellular cGAMP released by dead cells directly binds pmSTING and causes homodimerization and translocation of pmSTING from the plasmatic membrane to the perinuclear area, where it activates TBK1/IRF3/IFN signaling.