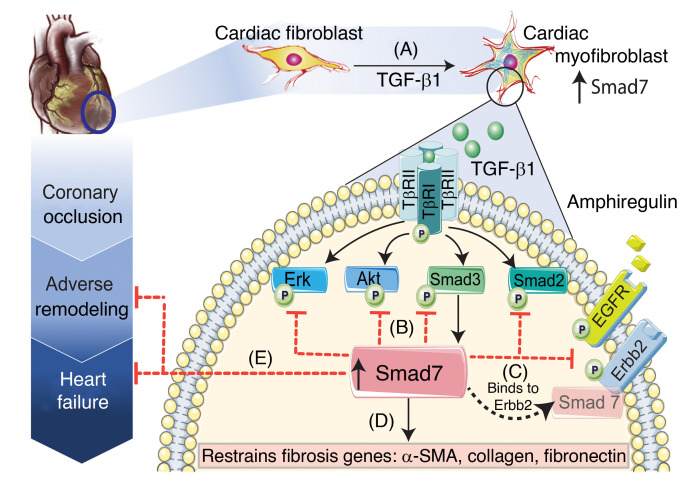
Figure 12. Schematic illustration of the findings of the study.
Our study shows that (A) in healing infarcts, the inhibitory Smad, Smad7, is overexpressed in activated myofibroblasts through a Smad3-dependent pathway. (B) Smad7 induction restrains TGF-β–mediated Smad2/3, Erk, and Akt signaling without affecting TβR activation. (C) In addition to its inhibitory effects on TGF-β cascades, Smad7 directly interacts with the receptor tyrosine kinase ErbB2 and restrains EGFR/ErbB2 activation in a TGF-β–independent manner. (D) Inhibition of both TGF-β and ErbB1/2 cascades by Smad7 restrains synthesis of structural and matricellular matrix–associated genes and attenuates myofibroblast conversion. (E) Myofibroblast-specific Smad7-mediated effects protect the infarcted heart from adverse remodeling and reduce heart failure–related mortality. Considering the role of ErbB2 in mediating sustained actions of other ErbBs in fibrotic conditions, the Smad7-ErbB2 interaction may amplify the antifibrotic effects of Smad7. Our findings suggest that Smad7 should be viewed beyond its role as a negative regulator of the TGF-β superfamily.