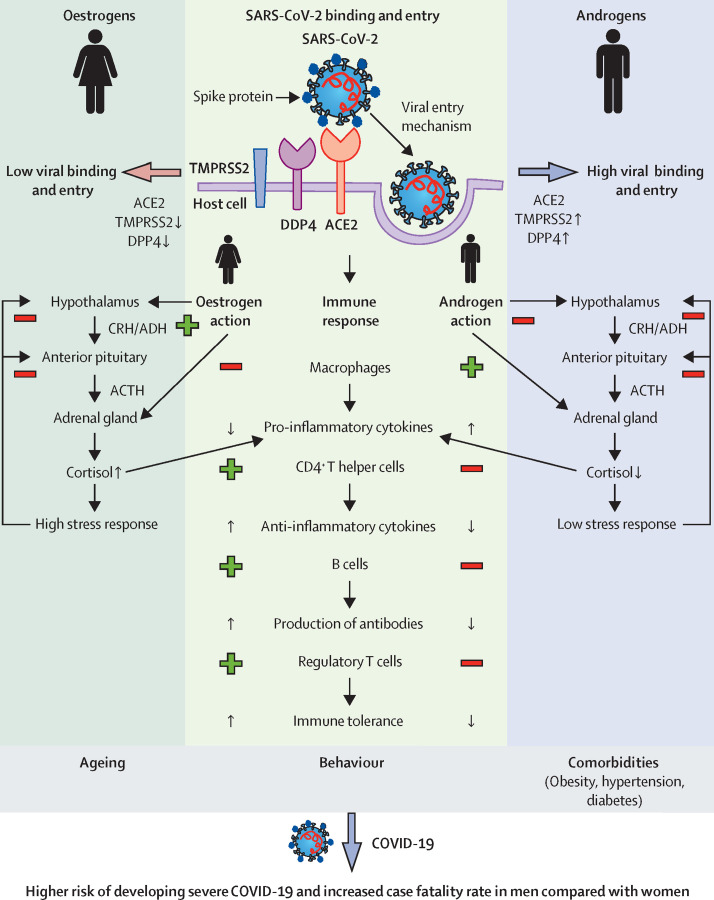
Figure 1.
Systemic and molecular basis for sexual dimorphism in COVID-19
SARS-CoV-2 uses its spike glycoprotein (S) to attach to the host cell, triggering fusion between virus and lipid membrane (exocytosis). ACE2, TMPRSS2, and DPP4 are critical mediators of this process and show tissue-specific and sex-specific expression patterns. Higher expression of TMPRSS2 and DDP4 in men is associated with increased viral binding and entry, leading to higher viral load compared with women. Oestrogens and androgens regulate the subsequent immune response. Women exhibit a stronger immune response, which is characterised by, among other factors, the release of anti-inflammatory cytokines (eg, IL-6, IL-1β, TNFα). In men, on the other hand, pro-inflammatory cytokines (eg, IL-4, IL-10) dominate. Sex-related differences in the response to stress further contribute to the predisposition of men to a pro-inflammatory state. An overall increased basal activity of the hypothalamic–pituitary–adrenal axis in women, mediated by oestrogens, results in increased cortisol concentrations compared with men. Decreased concentrations of anti-inflammatory cortisol further contribute to the pro-inflammatory status in men, possibly causing more severe COVID-19 courses in men compared with women. Other factors, such as age, smoking, alcohol consumption, and presence of comorbidities further contribute to the increased risk of severe disease and higher case fatality rates in men. Plus symbols mark an activation and minus symbols mark an inhibition. ACE2=angiotensin-converting enzyme 2. ACTH=adrenocorticotropic hormone. ADH=antidiuretic hormone. CRH=corticotropin-releasing hormone. DPP4=dipeptidyl peptidase-4. TMPRSS2=transmembrane protease serine subtype 2.