Summary
Background
Anemia is the most frequent hematologic abnormality among people living with human immunodeficiency virus (HIV) (PLWHIV) and is associated with HIV disease progression and higher risk of mortality of the patients. However, there is a wide variation of the prevalence of anemia among PLWHIV in different clinical settings. We aimed to obtain more precise estimates of prevalence of anemia and severity of anemia among PLWHIV, which may be important for patients, caregivers, researchers and health policy-makers.
Methods
We systematically searched PubMed, EMBASE, Web of Science, and Cochrane Library for original articles reporting the prevalence of anemia defined using age and sex-specific hemoglobin levels according to World Health Organization criteria among PLWHIV from inception to August 31, 2021. We used DerSimonian-Laird random-effects meta-analyses to obtain pooled prevalence and 95% confidence intervals (CIs) of anemia and severity of anemia among PLWHIV. A univariable meta-regression has been conducted to assess the association between anemia prevalence and study characteristics, including study design, median year of sampling, geographical region, World Bank Income level, and proportion of antiretroviral therapy (ART).
Findings
We included 63 observational studies covering 110,113 PLWHIV. The pooled prevalence of anemia was 39.7% (95% CI: 31.4%-48.0%) for children living with HIV aged <15 years, 46.6% (95% CI: 41.9%-51.4%) for adults (men and non-pregnant women) living with HIV aged ≥15 years, and 48.6% (95% CI: 41.6%-55.6%) for pregnant women living with HIV. Among adults living with HIV, the pooled prevalence of severity of anemia was 21.6% (95% CI: 19.9%-23.3%), 22.6% (95% CI: 14.8%-30.4%), and 6.2% (95% CI: 4.4%-8.1%) for mild, moderate and severe anemia, respectively. Compared with East Africa, anemia prevalence among adults living with HIV was higher in Southern Africa (p = 0.033).
Interpretation
Anemia is prevalent among PLWHIV. Thus, policies, strategies, and programs should be considered to identify the predictors of anemia among PLWHIV to reduce the burden of anemia among patients in the ART era.
Keywords: HIV, Anemia, Severity of anemia, Antiretroviral therapy
Research in context.
Evidence before this study
Anemia is the most frequent hematologic abnormalities among people living with human immunodeficiency virus (HIV) (PLWHIV); however, there is a wide variation of the prevalence in different clinical settings. Previous systematic reviews of prevalence of anemia among PLWHIV have focused on children below 18 years in specific regions or have included studies identifying anemia among PLWHIV not using age and sex-specific hemoglobin levels according to World Health Organization (WHO) criteria. Data are needed to establish the global burden of anemia among PLWHIV, these data are important for patients, caregivers, researchers and health policy-makers and are essential to inform normative guidance. We searched PubMed, EMBASE, Web of Science, and Cochrane Library for original articles reporting the prevalence of anemia from inception to August 31, 2021, using the term “(“HIV” or “human immunodeficiency virus”) and “anemia” and (“prevalence” or “incidence” or “epidemiology”)”. The classification of severity of anemia must be defined by using age and sex-specific Hb levels according to WHO criteria among PLWHIV. We identified 63 observational studies covering 110,113 PLWHIV for inclusion.
Added value of this study
To our knowledge, this current study is the first to assess the prevalence of anemia and severity of anemia among PLWHIV using age and sex-specific hemoglobin levels according to WHO criteria. We found that the pooled prevalence of anemia was 39.7% (95% CI: 31.4%−48.0%) for children living with HIV aged <15 years, 46.6% (95% CI: 41.9%−51.4%) for adults (men and non-pregnant women) living with HIV aged ≥15 years, and 48.6% (95% CI: 41.6%−55.6%) for pregnant women living with HIV. The pooled prevalence of severity of anemia was 21.6% (95% CI: 19.9%−23.3%), 22.6% (95% CI: 14.8%−30.4%), and 6.2% (95% CI: 4.4%−8.1%) for mild, moderate and severe anemia among adults living with HIV, respectively.
Implications of all the available evidence
Our findings clearly show that anemia is prevalent among PLWHIV, especially for adults and pregnant women living with HIV. There is also a need for clinicians to pay more attention to anemia among PLWHIV in the antiretroviral therapy era. The predictors of anemia among PLWHIV to reduce the burden of anemia among patients are warranted to be studied in the future.
Alt-text: Unlabelled box
Introduction
Globally, an estimated 37.6 million people in the world were living with human immunodeficiency virus (HIV) in 2020, and 1.5 million people were newly infected.1 Despite a steady but slow decline in the incidence rates of HIV infection over the past two decades, with improved survival due to the scale-up of antiretroviral therapies (ART), more people are living with HIV than ever before.2 However, in tandem with increases in life expectancy following the introduction of ART, hematological changes are one of the most common complications among PLWHIV and could impact both the length and quality of their lives.3, 4, 5 As the most common hematologic abnormality among PLWHIV, anemia has been cited as a prognostic marker for HIV disease progression and has been associated with reduced survival.5, 6, 7, 8
The causes of anemia among HIV-infected patients are multifactorial. HIV could directly and indirectly impact the survival and functioning of hematopoietic stem/progenitor cells (HSPCs) that reside in the bone marrow.9,10 In addition, the drugs used for ART, inflammatory mediators released during HIV infection and coinfections or opportunistic infections could also affect the proliferation and differentiation of HSPCs during hematopoiesis.9,10 Progressive depletion of HSPCs or suppression of their function could both result in hematologic abnormalities, such as anemia, thrombocytopenia, and neutropaenia.9,10 Of note, thrombocytopenia is often asymptomatic in HIV-infected patients and may be associated with a variety of bleeding abnormalities,11, 12, 13 which could possibly lead to an increased risk of anemia and even aggravate anemia.14
Over the past three decades, an increasing number of population-based studies have evaluated the prevalence of anemia and the possibly associated factors among PLWHIV; however, there was a wide variation in the prevalence of anemia ranging from 1.3% to 95% in different clinical settings.5,15,16 The possible explanations of the varied range of anemia prevalence among PLWHIV might be differences in criteria of anemia, progression of HIV disease, and ART status across previous studies.5,17, 18, 19, 20, 21, 22 For example, the prevalence of anemia (hemoglobin (Hb) concentration <12 g/dL for females and <13 g/dL for males) was 54.2% among PLWHIV entering pre-ART care (June 2011-June 2014) in Myanmar,21 which was higher than that among Chinese PLWHIV receiving ART (27.7%) in 2014.22 The prevalence of anemia that was diagnosed as Hb ≤10.5 g/dL was 42.8% among ART-naïve children living with HIV.20 Several previous reviews have indicated that anemia prevalence differed across studies using different definitions of anemia among PLWHIV, making comparisons difficult between studies.5,10,23 In addition, one of the previous reviews reported that three studies conducted in Africa using the same World Health Organization (WHO) definition reported a remarkably similar prevalence of anemia among PLWHIV.5 Therefore, we undertook a systematic review to estimate the prevalence of anemia and severity of anemia diagnosed using Hb levels according to WHO criteria for PLWHIV.24
Methods
Search strategy and selection criteria
We searched PubMed, EMBASE, Web of Science, and Cochrane Library for studies reporting the prevalence of anemia in HIV-infected people from inception to August 31, 2021, following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.25 The protocol of this study was not preregistered. Articles were identified using the search terms “(HIV” or “human immunodeficiency virus”) and “anemia” and (“prevalence” or “incidence” or “epidemiology”)”, without language restrictions. The detailed search strategies are presented in Supplementary Table 1.
The included studies were required to investigate PLWHIV and needed to present data that allowed us to calculate the prevalence of anemia. The prevalence of anemia and its severity were the primary and secondary outcomes of interest, and the included studies should be observational studies, including cross-sectional studies, longitudinal studies, and case control studies in which cases were representative samples of all HIV patients. Classification of severity of anemia must be determined by using age and sex-specific Hb levels according to WHO criteria24: no anemia (Hb ≥130 g/L for children 6–59 months of age, ≥115 g/L for children 5–11 years of age, ≥120 g/L for children 12–14 years of age, ≥120 g/L for non-pregnant women ≥15 years of age, ≥110 g/L for pregnant women, ≥130 g/L for men ≥15 years of age), mild anemia (100–109 g/L for children 6–59 months of age, 110–114 g/L for children 5–11 years of age, 110–119 g/L for children 12–14 years of age, 110–119 g/L for non-pregnant women ≥15 years of age, 100–109 g/L for pregnant women, 110–129 g/L for men ≥15 years of age), moderate anemia (70–99 g/L for children 6–59 months of age, 80–109 g/L for children 5–11 years of age, 80–109 g/L for children 12–14 years of age, 80–109 g/L for non-pregnant women ≥15 years of age, 70–99 g/L for pregnant women, 80–109 g/L for men ≥15 years of age), and severe anemia (<70 g/L for children 6–59 months of age, <80 g/L for children 5–11 years of age, <80 g/L for children 12–14 years of age, <80 g/L for non-pregnant women ≥15 years of age, <70 g/L for pregnant women, <80 g/L for men ≥15 years of age). We excluded studies if they were reviews, animal studies, or duplicate studies (enrolling the same population in the same region around the same period) with fewer sample; or if the diagnostic methods of anemia were unclear; if baseline sample were excluded due to anemia treated previously; baseline PLWHIV were not general patients but patients with WHO stage III or IV HIV disease, special risk groups (e.g., patients with tuberculosis, Kaposi sarcoma, or hepatitis C), or patients included with criteria that would be related to anemia (e.g., patients were screened for tuberculosis or without a current TB).
Two authors (GC and YW) independently performed the literature search, screened all abstracts and titles identified in the search, and assessed the full text considered potentially relevant. Any disagreements were resolved by discussion with a third reviewer (WJ).
Data analysis
Two authors (GC and YW) extracted information about the first author's last name, year of publication, geographical location, median year of sampling, population groups (children aged <15 years, adults (men and non-pregnant women aged ≥ 15 years), and pregnant women), sample size, mean (standard deviation, SD) or median (interquartile range, IQR) age of the study population, numbers of HIV patients diagnosed with anemia overall and stratified by severity of anemia, and proportion of ART at anemia diagnosis from every eligible study. The income level of each country was further classified into low-, low-middle-, upper-middle-, or high-income according to the World Bank's country classification.26 We extracted anemia cases at the first timepoint, when a cohort study reported several estimates of anemia cases at different timepoints, such as at baseline and after ART initiation. The two authors reached a consensus after discussing any controversial findings with a third author (WJ). The quality of the included studies was evaluated by a tool developed by Hoy and colleagues.27,28 Each study was assessed according to 10 items, and a score of one (yes) or zero (no) was assigned for each item. Quality was assessed on a 10-point tool and classified as low (>8), moderate (6–8), and high (≤5) risk of bias.
We performed DerSimonian-Laird random-effects meta-analyses of the included studies to calculate the pooled prevalence and 95% confidence intervals (CIs) of anemia and its severity in children, adults, and pregnant women, respectively. A univariable meta-regression was conducted to assess the association between anemia prevalence and several study characteristics, including study design, median year of sampling, geographical region, World Bank Income level and ART proportion. Statistical heterogeneity among the studies was estimated using the I2 statistic, and very low, low, moderate, and high degrees of heterogeneity were defined as ≤25%, 25% to ≤50%, 50% to ≤75%, and ≥75%, respectively.1 Publication bias was appraised using funnel plots and Egger's test for assessing asymmetry. All analyses were performed using Stata software (version 12.0; Stata SE Corporation LP, College Station, TX, USA). A two-sided p value <0.05 was considered statistically significant.29
Role of the funding source
The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Characteristics of included studies
A total of 4763 records were identified based on the initial search, of which 139 were selected for full-text evaluation after removal of duplications and initial screening (Figure 1). Among studies not using Hb levels according to WHO criteria to define anemia, most of them were more likely to not use age and sex-specific Hb levels or used a higher cutoff of for anemia and lower cutoff for severe anemia than that of WHO criteria.18,30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40 After applying the inclusion criteria, 63 studies (12 cohort, 2 case-control, and 49 cross-sectional studies) covering 110,113 PLWHIV met the inclusion criteria and were included in the meta-analysis6,17, 18, 19,21,36,41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97 (Table 1). In brief, the majority of the included studies were conducted in Africa (25 in East Africa, 8 in West and Central Africa, and 3 in Southern Africa),6,17, 18, 19,41, 42, 43,45,48,50,51,55, 56, 57, 58,60, 61, 62,65, 66, 67, 68,70, 71, 72, 73, 74,78,79,83, 84, 85,89,92,96,97 6 in Asia,44,46,47,49,52,53,63,64,76,80,82,87,90,91 5 in Europe,21,36,59,75,86 5 in North America,54,77,81,93,94 1 in Oceania,69 1 in Latin America & Caribbean,95 and 1 in multiple regions.88 PLWHIV were children aged <15 years in 9 studies,17,19,41, 42, 43, 44, 45, 46, 47 non-pregnant women and men aged ≥15 years in 51 studies,6,18,21,36,48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94 and pregnant women in 3 studies.95, 96, 97 All studies included in the current review were evaluated as having a moderate risk of bias (Supplementary Table 2).
Figure 1.
Study flowchart.
Note: PLWHIV=people living with human immunodeficiency virus; WHO=World Health Organization.
Table 1.
Characteristics of the studies included in the systematic review and meta-analysis.
| First author, year (Country) | Median year of sampling (y) | Study design | Population group | Sample size, n | Age, y | Anemia cases by severity, n |
Proportion of ART,% | Region | World Bank Income level | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Mild | Moderate | Severe | |||||||||
| Bayleyegn et al., 2021 (Ethiopia)41 | 2020 | Cross-sectional | Children | 255 | Median (IQR): 13 (10–14) | 54 | 99.2 | East Africa | Low | |||
| Geleta et al., 2021 (Ethiopia)17 | 2018 | Cross-sectional | Children | 256 | Median (IQR): 12 (10–14) | 98 | 100.0 | East Africa | Low | |||
| Melku et al., 2020 (Ethiopia)42 | 2011 | Cross-sectional | Children | 200 | NA | 75 | 41 | 34 | 100.0 | East Africa | Low | |
| Tsegay et al., 2017 (Ethiopia)43 | 2013 | Cross-sectional | Children | 224 | Median (IQR): 8 (NA) | 66 | 50.0 | East Africa | Low | |||
| Ahumareze et al., 2016 (Nigeria)19 | 2015 | Cross-sectional | Children | 164 | Mean (SD): 8.3 (1.9) | 89 | 2 | 100.0 | West and Central Africa | Lower-middle | ||
| Shet et al., 2015 (India)44 | 2011 | Cohort | Children | 240 | Mean (SD): 7.7 (2.6) | 113 | 16 | 43.3 | Asia | Lower-middle | ||
| Ezeonwu et al., 2014 (Nigeria)45 | 2005 | Cross-sectional | Children | 67 | NA | 2 | 71.6 | West and Central Africa | Lower-middle | |||
| Kapavarapu et al., 2012 (India)46 | 2010 | Cross-sectional | Children | 85 | Mean (SD): 9.2 (NA) | 34 | 29.4 | Asia | Lower-middle | |||
| Shet et al., 2012 (India)47 | 2008 | Cross-sectional | Children | 80 | Mean (SD): 6.8 (NA) | 42 | 4 | 36.3 | Asia | Lower-middle | ||
| Damtie et al., 2021 (Ethiopia)48 | 2020 | Cross-sectional | Adult | 334 | Mean (SD): 38.8 (9.9) | 124 | 0.0 | East Africa | Low | |||
| Butt et al., 2020 (Japan)59 | 2016 | Cross-sectional | Adult | 230 | Mean (SD): 38.0 (14.5) | 152 | 26 | NA | Asia | High | ||
| Baye et al., 2020 (Ethiopia)50 | 2019 | Cross-sectional | Adult | 392 | Mean (SD): 40.5 (8.5) | 191 | 94.6 | East Africa | Low | |||
| Berhane et al., 2020 (Ethiopia)51 | 2019 | Case-control | Adult | 212 | Mean (SD): 41.68 (10.61) | 80 | 0.0 | East Africa | Low | |||
| Dobe et al., 2020 (Mozambique)18 | 2013 | Cross-sectional | Adult | 264 | Mean (SD): 39.3 (9.8) | 153 | 100.0 | Southern Africa | Low | |||
| Khatri et al., 2020 (Nepal)52 | 2017 | Cross-sectional | Adult | 350 | Mean (SD): 38.9 (9.1) | 112 | 100.0 | Asia | Lower-middle | |||
| Sah et al., 2020 (Nepal)53 | 2017 | Cross-sectional | Adult | 210 | Mean (SD): 37.50 (10.57) | 140 | 30 | 85 | 25 | 81.9 | Asia | Lower-middle |
| Zanni et al., 2020 (US)54 | 2002 | Cross-sectional | Adult | 1449 | Median (IQR): 49 (45–55) | 348 | 100.0 | North America | High | |||
| Albrecht et al., 2019 (Tanzania) 6 | 2015 | Cohort | Adult | 1622 | Median (IQR): 38 (31–46) | 288 | 100.0 | East Africa | Lower-middle | |||
| Ageru et al., 2019 (Ethiopia) 55 | 2016 | Cross-sectional | Adult | 411 | Mean (SD): 35.0 (8.9) | 150 | 106 | 40 | 4 | 74.9 | East Africa | Low |
| Tamir et al., 2019 (Ethiopia) 56 | 2016 | Cross-sectional | Adult | 402 | Mean (SD): 36.2 (9.5) | 175 | 69 | 70 | 36 | 0.0 | East Africa | Low |
| Yesuf et al., 2019 (Ethiopia)57 | 2012 | Cross-sectional | Adult | 404 | NA | 133 | 0.0 | East Africa | Low | |||
| Ezeamama et al., 2018 (Uganda)58 | 2009 | Cohort | Adult | 398 | Mean (SD): 35.8 (9.0) | 194 | 113 | 50.0 | East Africa | Low | ||
| Hentzien et al., 2018 (France)59 | 2008 | Cross-sectional | Adult | 1415 | Mean (SD): 65.7 (5.5) | 296 | 88.2 | Europe | High | |||
| Katemba et al., 2018 (Uganda) 60 | 2016 | Cross-sectional | Adult | 141 | Mean (SD): 34 (NA) | 95 | 0.0 | East Africa | Low | |||
| Beyene et al., 2017 (Ethiopia) 61 | 2013 | Cross-sectional | Adult | 528 | Mean (SD): 33.69 (9.08) | 227 | 37 | 0.0 | East Africa | Low | ||
| Fiseha et al., 2017 (Ethiopia)62 | 2012 | Cross-sectional | Adult | 373 | Mean (SD): 34.6 (10.8) | 128 | 76 | 46 | 6 | 0.0 | East Africa | Low |
| Jin et al., 2017a (China)63 | 2012 | Cross-sectional | Adult | 8632 | Mean (SD): 47.0 (8.3) | 101 | 100.0 | Asia | Upper-middle | |||
| Jin et al., 2017b (China)64 | 2014 | Cross-sectional | Adult | 9402 | Mean (SD): 47.8 (8.5) | 2534 | 1788 | 495 | 251 | 100.0 | Asia | Upper-middle |
| Gunda et al., 2017a (Tanzania)65 | 2016 | Cohort | Adult | 740 | Median (IQR): 35 (27–42) | 288 | 53 | 100.0 | East Africa | Lower-middle | ||
| Gunda et al., 2017b (Tanzania)66 | 2015 | Cross-sectional | Adult | 1205 | Median (IQR): 41 (32–48) | 704 | 0.0 | East Africa | Lower-middle | |||
| Melese et al., 2017 (Ethiopia)67 | 2015 | Cross-sectional | Adult | 377 | Mean (SD): 35.21 (9.27) | 290 | 62.9 | East Africa | Low | |||
| Sahle et al., 2017 (Ethiopia)68 | 2014 | Cross-sectional | Adult | 172 | Mean (SD): 31.95 (7.6) | 89 | 82.6 | East Africa | Low | |||
| Widiyanti et al., 2017 (Papua New Guinea)69 | 2015 | Cross-sectional | Adult | 90 | NA | 50 | 100.0 | Oceania | Lower-middle | |||
| Gunda et al., 2016 (Tanzania)70 | 2012 | Cross-sectional | Adult | 346 | Median (IQR): 41.2 (19–66) | 245 | 105 | 91 | 49 | 100.0 | East Africa | Lower-middle |
| Obirikorang et al. 2016 (Ghana)71 | 2013 | Cross-sectional | Adult | 319 | Mean (SD): 38.9 (9.9) | 76 | 41 | 19 | 16 | 68.7 | West and Central Africa | High |
| Oo et al., 2016 (France)21 | 2013 | Cross-sectional | Adult | 11,454 | Mean (SD): 37 (10) | 6420 | 2184 | 3285 | 951 | 0.0 | Europe | High |
| Akinyemi et al., 2015 (Nigeria)72 | 2009 | Cross-sectional | Adult | 14,857 | Mean (SD): 36.4 (10.2) | 1846 | 45.1 | West and Central Africa | Lower-middle | |||
| Akilimali et al., 2015 (Democratic Republic of Congo)73 | 2008 | Cohort | Adult | 756 | Mean (SD): 39.5 (9.8) | 528 | 201 | 278 | 49 | 0.0 | West and Central Africa | Low |
| Kerkhoff et al., 2015 (South Africa)74 | 2004 | Cohort | Adult | 1521 | Median (IQR): 33 (28–33) | 1203 | 304 | 469 | 49 | 0.0 | Southern Africa | Upper-middle |
| Minchella et al., 2015 (UK)75 | 1997 | Cross-sectional | Adult | 196 | Mean (SD): 34.3 (9.8) | 110 | NA | Europe | High | |||
| Mijiti et al., 2015 (China)76 | 2009 | Cross-sectional | Adult | 2252 | Mean (SD): 36.6 (8.3) | 875 | 433 | 383 | 59 | 0.0 | Asia | Upper-middle |
| Erqou et al., 2014 (US)77 | 2004 | Cross-sectional | Adult | 8039 | Mean (SD): 50.0 (7.3) | 3933 | NA | North America | High | |||
| Kerkhoff et al., 2014 (South Africa)78 | 2004 | Cross-sectional | Adult | 814 | Median (IQR): 33 (29–39) | 574 | 228 | 310 | 36 | 0.0 | Southern Africa | Upper-middle |
| Kyeyune et al., 2014 (Uganda)79 | 2011 | Cross-sectional | Adult | 400 | Mean (SD): 36.0 (9.0) | 191 | 50.0 | East Africa | Low | |||
| Martin et al., 2014 (Nepal)80 | 2011 | Cross-sectional | Adult | 319 | Mean (SD): 35.6 (6.9) | 178 | 82 | 84 | 12 | 73.1 | Asia | Lower-middle |
| Santiago et al., 2014 (US)81 | 2005 | Cohort | Adult | 1486 | Median (IQR): 40 (21–79) | 616 | 64.4 | North America | High | |||
| Shet et al., 2014 (India)82 | 2010 | Cohort | Adult | 321 | Mean (SD): 37 (8) | 82 | 0.0 | Asia | Lower-middle | |||
| Tesfaye et al., 2014 (Ethiopia)83 | 2010 | Cross-sectional | Adult | 349 | Mean (SD): 34.6 (8.5) | 74 | 0.0 | East Africa | Low | |||
| Ferede et al., 2013 (Ethiopia)84 | 2012 | Cross-sectional | Adult | 420 | NA | 138 | 10 | 0.0 | East Africa | Low | ||
| Hadgu et al., 2013 (Ethiopia)85 | 2012 | Cross-sectional | Adult | 376 | Mean (SD): 32.5 (8) | 250 | 100.0 | East Africa | Low | |||
| Khasanova et al., 2013 (Russia)86 | NA | Cross-sectional | Adult | 99 | NA | 30 | NA | Europe | Upper-middle | |||
| Liu et al., 2013 (China)87 | 2012 | Cross-sectional | Adult | 276 | Mean (SD): 46.7 (10.4) | 74 | 4 | 94.9 | Asia | Upper-middle | ||
| Zhou et al., 2013 (Western Africa, Southern Africa, Eastern Africa, Central Africa, Asian-Pacific, Caribbean, Central America, and South America)88 | NA | Cohort | Adult | 19,947 | Median (IQR): 37 (31–44) | 6502 | 100.0 | – | – | |||
| Akinbo et al., 2012 (Nigeria)89 | 2011 | Cross-sectional | Adult | 285 | NA | 129 | 100.0 | West and Central Africa | Lower-middle | |||
| Meidani et al., 2012 (Iran)90 | 2010 | Cross-sectional | Adult | 212 | Mean (SD): 36.2 (9.1) | 95 | 100.0 | Asia | Lower-middle | |||
| De Santis et al., 2011 (France)36 | 2009 | Cross-sectional | Adult | 701 | Mean (SD): 42 (NA) | 263 | 82.3 | Europe | High | |||
| Subbaraman et al., 2009 (India)91 | 2001 | Cross-sectional | Adult | 6996 | Mean (SD): 33.6 (8.3) | 4792 | NA | Asia | Lower-middle | |||
| Erhabor et al., 2006 (Nigeria)92 | NA | Case-control | Adult | 100 | Mean (SD): 35.2 (1.29) | 43 | NA | West and Central Africa | Lower-middle | |||
| Berhane et al., 2004 (US)93 | 1994 | Cohort | Adult | 2056 | Median (IQR): 36 (NA) | 761 | 100.0 | North America | High | |||
| Semba et al., 2001 (US)94 | 1994 | Cohort | Adult | 361 | Mean (SD): 38.1 (NA) | 140 | 52.1 | North America | High | |||
| Delicio et al., 2018 (Brazil)95 | 2007 | Cohort | Pregnant women | 793 | Median (IQR): 28 (NA) | 396 | 100.0 | Latin America & Caribbean | Upper-middle | |||
| Manyanga et al., 2014 (Tanzania)96 | 2013 | Cross-sectional | Pregnant women | 420 | Mean (SD): 28 (5.2) | 227 | 21 | 100.0 | East Africa | Lower-middle | ||
| Ezechi et al., 2013 (Nigeria)97 | 2008 | Cross-sectional | Pregnant women | 2318 | Mean (SD): 30.5 (3.5) | 985 | 37.1 | West and Central Africa | Lower-middle | |||
Note: ART=antiretroviral therapy; IQR=interquartile range; NA=not available; SD=standard deviation; UK=United Kingdom; US=United States.
Prevalence of anemia among children living with HIV
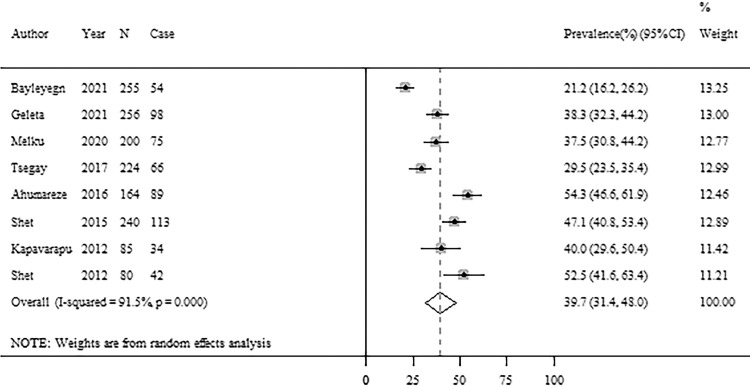
Among all the included studies, 9 studies reported cases of anemia or severity of anemia among children living with HIV aged <15 years (Table 1). Figure 2 presents the forest plot of the prevalence of anemia among children living with HIV. The pooled prevalence of anemia was 39.7% (95% CI: 31.4%−48.0%) from 8 studies, but with high heterogeneity (I2=91.5%, p <0.001) (Figure 2). There was no evidence of publication bias based on the funnel plot and Egger's test (Supplementary Figure 1). For the severity of anemia, 1 study reported both mild and moderate anemia cases and 4 studies reported severe anemia cases among children living with HIV. The pooled prevalence of severe anemia was 3.7% (95% CI: 0.8%−6.7%) with moderate heterogeneity between studies (Supplementary Figure 2) and no evidence of publication bias (Supplementary Figure 3).
Figure 2.
Forest plots of anemia prevalence among children living with HIV.
Note: CI=confidence interval; HIV=human immunodeficiency virus.
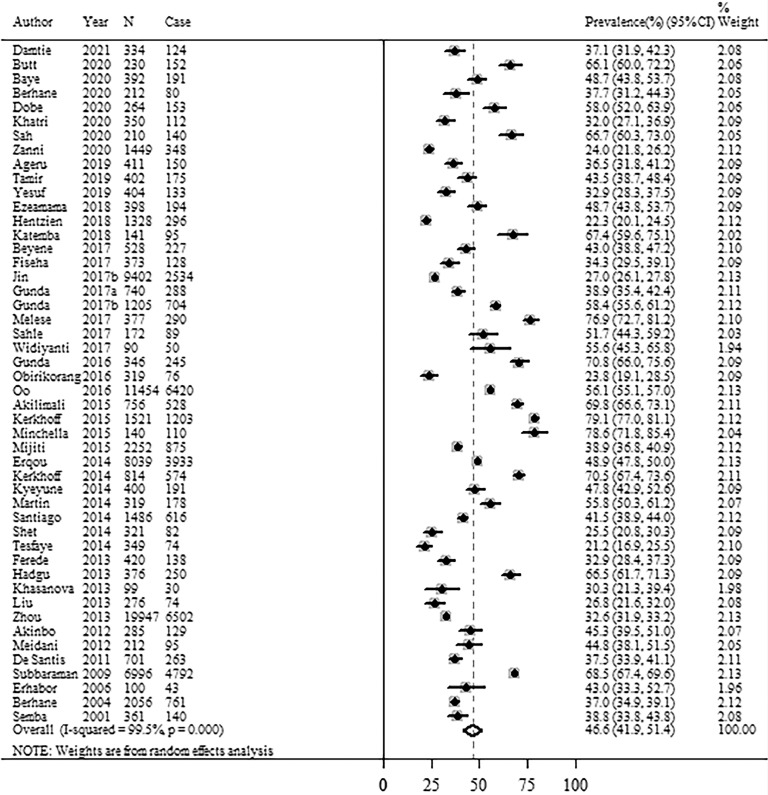
Prevalence of anemia among adults living with HIV
Figure 3 shows the prevalence of anemia among adults living with HIV aged >15 years from 47 studies included in this study. The overall estimate of anemia prevalence was 46.6% (95% CI: 41.9%−51.4%) among adults living with HIV (Figure 3). The I2 statistic (99.5%, p <0.001) indicated substantial heterogeneity between the studies (Figure 3). The funnel plot and Egger's test did not detect any publication bias (Supplementary Figure 4). Across the severity of anemia, the pooled prevalence of anemia was 21.6% (95% CI: 19.9%−23.3%) for mild anemia from 14 studies, 22.6% (95% CI: 14.8%−30.4%) for moderate anemia from 13 studies, and 6.2% (95% CI: 4.4%−8.1%) for severe anemia from 21 studies, but with high heterogeneity (Supplementary Figure 5). No publication bias was found for the pooled prevalence of severity of anemia based on the funnel plot and Egger's test (Supplementary Figs. 6–8).
Figure 3.
Forest plots of anemia prevalence among adults living with HIV.
Note: CI=confidence interval; HIV=human immunodeficiency virus.
Prevalence of anemia among pregnant women living with HIV
This study included 3 studies that reported anemia cases among pregnant women living with HIV (Table 1). The pooled prevalence of anemia was 48.6% (95% CI: 41.6%−55.6%), but with high heterogeneity (I2=92.6%, p <0.001) (Figure 4). No visual publication bias was found based on the funnel plot (Supplementary Figure 9).
Figure 4.
Forest plots of anemia prevalence among pregnant women living with HIV.
Note: CI=confidence interval; HIV=human immunodeficiency virus.
Meta-regression of the association between anemia prevalence and study characteristics
Table 2 shows the results of the univariable meta-regression analysis of the association between anemia prevalence and study characteristics. The univariable analyses showed that geographic region was significantly associated with anemia prevalence among adults living with HIV, leading the between-study heterogeneity to be explained by 4.15%. The prevalence of anemia among PLWHIV was higher across studies conducted in Southern Africa than in East Africa (b = 22.22; 95% CI: 1.93, 42.51). The study design, median year of sampling, World Bank Income level, and ART proportion were not significantly associated with the prevalence of anemia among adults living with HIV.
Table 2.
Univariable meta-regression of anemia prevalence and study characteristics among adults living with HIV.
| Study Characteristic | Number of studies, n | Coefficient (95% CI) | p value | R2 |
|---|---|---|---|---|
| Study design | −3.41% | |||
| Cross-sectional | 36 | Ref. | ||
| Cohort | 10 | −2.57 (−14.60, 9.47) | 0.669 | |
| Case-control | 2 | −7.15 (−32.16, 17.87) | 0.677 | |
| Median year of sampling (y) | 45 | 0.06 (−1.22, 1.34) | 0.922 | −2.36% |
| Geographic region | 4.15% | |||
| East Africa | 19 | Ref. | ||
| West and Central Africa | 4 | −1.48 (−19.62, 16.66) | 0.870 | |
| Southern Africa | 3 | 22.22 (1.93, 42.51) | 0.033 | |
| Asia | 10 | −1.94 (−14.74, 10.86) | 0.761 | |
| Europe | 5 | −2.17 (−18.67, 14.33) | 0.792 | |
| North America | 5 | −9.03 (−25.40, 7.33) | 0.271 | |
| Other | 1 | 8.48 (−26.40, 43.36) | 0.490 | |
| World Bank Income level | −4.39% | |||
| Low-income | 19 | Ref. | ||
| Lower-middle-income | 11 | 2.18 (−10.77, 15.13) | 0.736 | |
| Upper-middle-income | 6 | −2.23 (−18.18, 13.73) | 0.780 | |
| High-income | 11 | −4.85 (−17.75, 8.04) | 0.452 | |
| ART proportion,% | −3.78% | |||
| <20 | 16 | Ref. | ||
| 20–79.9 | 9 | −0.32 (−14.10, 13.45) | 0.963 | |
| ≥80 | 17 | −3.63 (−15.16, 7.89) | 0.528 |
Note: ART=antiretroviral therapy; CI=confidence interval.
Discussion
To the best of our knowledge, this current study is the first updated and comprehensive systematic review and meta-analysis of the prevalence of anemia and severity of anemia diagnosed with age and sex-specific Hb levels using WHO criteria among PLWHIV. The standardized definitions of anemia and its severity reduced heterogeneity largely because of methodologic variability and made the synthesis of prevalence possible. In addition, a meaningful standardized definition of anemia will provide the basis for better comparisons regarding the prevalence and clinical outcomes of anemia among PLWHIV.5 Based on data from 63 observational studies covering 110,113 PLWHIV across all population groups, we found that the pooled prevalence of anemia was 39.7% for children, 46.6% for adults, and 48.6% for pregnant women living with HIV. This study found a higher prevalence of anemia among adults living with HIV in Southern Africa than in East Africa.
Our findings corroborated previously published evidence that anemia was prevalent among all population groups of PLWHIV.5,10,98,99 HIV infects bone marrow stromal cells; however, it remains unclear to what extent hematopoietic progenitors are susceptible to HIV infection.100,101 Moreover, HIV disrupts the bone marrow microenvironment and causes cytokine imbalance, affecting hematopoietic progenitor cells in different ways.10 Several factors may also play a role in the development of anemia in PLWHIV, including opportunistic infections, chronic diseases, nutritional deficiencies, and toxicities from medications.8,10,102 PLWHIV suffer from frequent bacterial, viral, and fungal infections, which are an important cause of anemia development and some of these infections are traditionally related to abnormal hematopoiesis.5,100 For example, two previous studies included in this study reported a much higher prevalence of severe anemia among PLWHIV with Kaposi sarcoma103 and tuberculosis.104 Anemia may also be caused by drugs administered, either ART agents or agents to treat infection or lymphoma among PLWHIV.9,100 This current review did not include studies defining anemia without using age and sex-specific Hb levels according to WHO criteria, which reported the prevalence of anemia ranging from 9.2% to 85.5%,20,30,32, 33, 34, 35,37, 38, 39,105, 106, 107, 108, 109, 110, 111 and the prevalence of severity of anemia ranging from 7.6% to 52.4% for mild anemia,32,38,105,106 1.9% to 76.1% for moderate anemia,31,32,38,105 and 0.2% to 29.1% for severe anemia.31, 32, 33,37,38,105,112 There were also differences in the definitions of anemia within these excluded studies, such as using different Hb cutoffs, or not using age and sex-specific Hb cutoffs, making a comparison of the results between studies difficult.
In line with previous reviews,10,113 this study found that the prevalence of anemia varied across countries and was higher in African regions. The differences in the prevalence of anemia across regions could be explained by the differences in the levels of poverty, malnutrition and the overall poor economic state, which are accentuated mainly in African countries.10,113 In addition, we found that anemia was prevalent among adults living with HIV irrespective of ART proportion. Previous studies have reported that ART may have a protective effect on the development of anemia, through reduced disease progression.8,71,114 Moreover, ART could improve the immunity of PLWHIV by decreasing the occurrence of multiple opportunistic infections, which are identified to potentially cause anemia.8,102 However, ART drugs also have adverse side effects, which could cause anemia among PLWHIV.10,115 There are ART drugs that could worsen anemia among PLWHIV, such as stavudine and zidovudine.116,117 The association between ART and anemia among people living with HIV warrants further study. Previous evidence has shown that anemia is an established adverse prognostic marker and HIV-infected patients with anemia have a greater risk for mortality than patients without anemia.118 Thus, prompt identification of anemia may result in improved morbidity and mortality of patients initiating ART. Anemia of chronic disease could be ameliorated if recurrent infections are prevented, either by prophylactic use of antimicrobial therapy or by improvement of the immune deficiency by effective ART among PLWHIV. For acute, severe, or life-threatening anemia, the standard treatment is blood transfusion, and the main advantage of this modality compared with drug therapy is rapid recovery from anemia with relief of symptoms. Thus, there is a need for regular screening of hematological parameters among PLWHIV and providing treatment, which could be crucial for reducing the advancement of HIV disease and its subsequent hematological complications in the ART era.
This current systematic review and meta-analysis included studies using Hb levels according to WHO criteria to define anemia and the severity of anemia, making the results of the included studies comparable and producing reliably pooled estimates in this study. However, some limitations in this study should also be acknowledged. First, there is the possibility of duplicate PLWHIV within studies. We included one study with a large sample of PLWHIV from multiple countries88; thus, duplicate patients may potentially exist within the included studies. Second, the high heterogeneity between the studies reduced the precision of our pooled effect size estimates, urging some caution in our interpretation of the prevalence of anemia. Third, the limited number of included studies for other common causes of anemia among PLWHIV increased the uncertainty of our pooled prevalence estimates, and the sources of heterogeneity could only be explored by subgroup analysis in a limited set of population groups. Finally, unmeasured or residual confounding in the source studies could not be addressed in this meta-analysis using only published data.
In conclusion, this systematic review and meta-analysis found that anemia was prevalent among PLWHIV. Thus, clinicians should pay more attention to anemia among PLWHIV in the ART era. Policies, strategies, and programs should be considered to identify the predictors of anemia among PLWHIV to reduce the burden of anemia among patients.
Data sharing statement
All data used in this manuscript can be found in the online versions of the studies that were accessed. Our own data synthesis of these manuscripts is available upon reasonable request.
Contributors
GC and ML were responsible for the conception and design of the study; GC, YW and WJ performed data acquisition; GC performed data analyses; GC and ML interpreted the results; GC drafted the manuscript; and all authors critically revised the manuscript and approved the final version.
Declaration of interests
Authors declare no conflict of interest with the content of this article.
Funding
This work was supported by the National Natural Science Foundation of China (grant number: 71934002, 71874003) and National Key Research and Development Program of China (grant number: 2020YFC0846300).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101283.
Appendix. Supplementary materials
References
- 1.Higgins J.P., Thompson S.G., Deeks J.J., et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joint United Nations Programme on HIV/AIDS (UNAIDS) UNAIDS; Geneva, Switzerland: 2020. UNAIDS Data 2020.https://www.unaids.org/en/resources/documents/2020/unaids-data Available at: Accessed 5 July 2021. [Google Scholar]
- 3.Ezeamama A.E., Sikorskii A., Bajwa R.K., et al. Evolution of anemia types during antiretroviral therapy-implications for treatment outcomes and quality of life among HIV-infected adults. Nutrients. 2019;11:4. doi: 10.3390/nu11040755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore R.D. Human immunodeficiency virus infection, anemia, and survival. Clin Infect Dis. 1999;29(1):44–49. doi: 10.1086/520178. [DOI] [PubMed] [Google Scholar]
- 5.Belperio P.S., Rhew D.C. Prevalence and outcomes of anemia in individuals with human immunodeficiency virus: a systematic review of the literature. Am J Med. 2004;116 (Suppl 7A):27s–43s. doi: 10.1016/j.amjmed.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 6.Albrecht S., Franzeck F.C., Mapesi H., et al. Age-related comorbidities and mortality in people living with HIV in rural Tanzania. AIDS. 2019;33(6):1031–1041. doi: 10.1097/QAD.0000000000002171. [DOI] [PubMed] [Google Scholar]
- 7.Haider B.A., Spiegelman D., Hertzmark E., et al. Anemia, iron deficiency, and iron supplementation in relation to mortality among HIV-infected patients receiving highly active antiretroviral therapy in Tanzania. Am J Trop Med Hyg. 2019;100(6):1512–1520. doi: 10.4269/ajtmh.18-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Volberding P.A., Levine A.M., Dieterich D., et al. Anemia in HIV infection: clinical impact and evidence-based management strategies. Clin Infect Dis. 2004;38(10):1454–1463. doi: 10.1086/383031. [DOI] [PubMed] [Google Scholar]
- 9.Durandt C., Potgieter Jc, Fau - Mellet J., et al. HIV and haematopoiesis. S Afr Med J. 2019;109(8b):40–45. doi: 10.7196/SAMJ.2019.v109i8b.13829. [DOI] [PubMed] [Google Scholar]
- 10.Marchionatti A., Parisi M.M. Anemia and thrombocytopenia in people living with HIV/AIDS: a narrative literature review. Int Health. 2021;13(2):98–109. doi: 10.1093/inthealth/ihaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scaradavou A. HIV-related thrombocytopenia. Blood Rev. 2002;16(1):73–76. doi: 10.1054/blre.2001.0188. [DOI] [PubMed] [Google Scholar]
- 12.Sloand E.M., Klein H.G., Banks S.M., et al. Epidemiology of thrombocytopenia in HIV infection. Eur J Haematol. 1992;48(3):168–172. doi: 10.1111/j.1600-0609.1992.tb00591.x. [DOI] [PubMed] [Google Scholar]
- 13.Vinholt P.J. The role of platelets in bleeding in patients with thrombocytopenia and hematological disease. Clin Chem Lab Med. 2019;57(12):1808–1817. doi: 10.1515/cclm-2019-0380. [DOI] [PubMed] [Google Scholar]
- 14.Moyle G. Anaemia in persons with HIV infection: prognostic marker and contributor to morbidity. AIDS Rev. 2002;4(1):13–20. [PubMed] [Google Scholar]
- 15.Moore R.D., Creagh-Kirk T., Keruly J., et al. Long-term safety and efficacy of zidovudine in patients with advanced human immunodeficiency virus disease. Zidovudine epidemiology study group. Arch Intern Med. 1991;151(5):981–986. [PubMed] [Google Scholar]
- 16.Treacy M., Lai L., Costello C., et al. Peripheral blood and bone marrow abnormalities in patients with HIV related disease. Br J Haematol. 1987;65(3):289–294. doi: 10.1111/j.1365-2141.1987.tb06855.x. [DOI] [PubMed] [Google Scholar]
- 17.Geleta M.L., Solomon F.B., Tufa E.G., et al. Predictors of anemia among HIV-infected children on antiretroviral therapy in wolaita zone, South Ethiopia: a facility-based cross-sectional study. HIV Aids Res Palliat Care. 2021;13:13–19. doi: 10.2147/HIV.S282845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dobe I., Manafe N., Majid N., et al. Patterns of cardiovascular risk and disease in HIV-positive adults on anti-retroviral therapy in Mozambique. Cardiovasc J Afr. 2020;31(4):190–195. doi: 10.5830/CVJA-2020-007. [DOI] [PubMed] [Google Scholar]
- 19.Ahumareze R.E., Rankin J., David A., et al. Prevalence of anaemia and the relationship between haemoglobin concentration and CD4 count in HIV positive children on highly active antiretroviral therapy (HAART) in Lagos, Nigeria. Curr Pediatr Res. 2016;20(1–2):29–36. [Google Scholar]
- 20.Geletaw T., Tadesse M.Z., Demisse A.G. Hematologic abnormalities and associated factors among HIV infected children pre- and postantiretroviral treatment, North West Ethiopia. J Blood Med. 2017;8:99–105. doi: 10.2147/JBM.S137067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oo M.M., Gupta V., Aung T.K., et al. Alarming attrition rates among HIV-infected individuals in pre-antiretroviral therapy care in Myanmar, 2011-2014. Glob Health Action. 2016;9:31280. doi: 10.3402/gha.v9.31280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Z., Jin Y., Li P., et al. Prevalence and influencing factors of anemia in elderly patients with HIV/AIDS. Chin J Dermatovenereol. 2017;31(4):442–444. [Google Scholar]
- 23.Wagnew F., Eshetie S., Alebel A., et al. Burden of anemia and its association with HAART in HIV infected children in Ethiopia: a systematic review and meta-analysis. BMC Infect Dis. 2019;19(1):1032. doi: 10.1186/s12879-019-4656-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO . World Health Organization; Geneva: 2011. Haemoglobin Concentrations For the Diagnosis of Anaemia and Assessment of severity. Vitamin and Mineral Nutrition Information System.http://www.who.int/vmnis/indicators/haemoglobin.pdf (WHO/NMH/NHD/MNM/11.1) Accessed 20 May 2021. [Google Scholar]
- 25.Moher D., Liberati A., Tetzlaff J., et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The World Bank . The World Bank; 2020. World Bank Country and Lending Groups.https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups Accessed 1 December 2021. [Google Scholar]
- 27.Hoy D., Brooks P., Woolf A., et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65(9):934–939. doi: 10.1016/j.jclinepi.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 28.Mogire R.M., Mutua A., Kimita W., et al. Prevalence of vitamin D deficiency in Africa: a systematic review and meta-analysis. Lancet Glob Health. 2020;8(1):e134–e142. doi: 10.1016/S2214-109X(19)30457-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Egger M., Davey Smith G., Schneider M., et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demitto F.O., Araujo-Pereira M., Schmaltz C.A., et al. Impact of persistent anemia on systemic inflammation and tuberculosis outcomes in persons living with HIV. Front Immunol. 2020;11:588405. doi: 10.3389/fimmu.2020.588405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ban L., Chen Y., Kong Q., et al. Clinical and laboratory diagnosis about fungal infection in 611 AIDS. Chin J Aids STD. 2020;26(11):1154–1156. ,1173. [Google Scholar]
- 32.Bate A., Kimbi H.K., Lum E., et al. Malaria infection and anaemia in HIV-infected children in Mutengene, Southwest Cameroon: a cross sectional study. BMC Infect Dis. 2016;16(1) doi: 10.1186/s12879-016-1853-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Renner L.A., Dicko F., Kouéta F., et al. Anaemia and zidovudine-containing antiretroviral therapy in paediatric antiretroviral programmes in the IeDEA Paediatric West African Database to evaluate AIDS. J Int AIDS Soc. 2013;16(1):18024. doi: 10.7448/IAS.16.1.18024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nansera D., Bajunirwe F., Elyanu P., et al. Mortality and loss to follow-up among tuberculosis and HIV co-infected patients in rural southwestern Uganda. Int J Tuberc Lung Dis. 2012;16(10):1371–1376. doi: 10.5588/ijtld.11.0589. [DOI] [PubMed] [Google Scholar]
- 35.Mocroft A., Lifson A.R., Touloumi G., et al. Haemoglobin and anaemia in the SMART study. Antivir Ther. 2011;16(3):329–337. doi: 10.3851/IMP1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Santis G.C., Brunetta D.M., Vilar F.C., et al. Hematological abnormalities in HIV-infected patients. Int J Infect Dis. 2011;15(12):e808–e811. doi: 10.1016/j.ijid.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 37.Mata-Marin J.A., Gaytan-Martinez J.E., Martinez-Martinez R.E., et al. Risk factors and correlates for anemia in HIV treatment-naive infected patients: a cross-sectional analytical study. BMC Res Notes. 2010;3:230. doi: 10.1186/1756-0500-3-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sullivan P.S., Hanson D.L., Brooks J.T. Impact on hemoglobin of starting combination antiretroviral therapy with or without zidovudine in anemic HIV-infected patients. JAIDS J Acquir Immune Defic Syndr. 2008;48(2):163–168. doi: 10.1097/QAI.0b013e3181685714. [DOI] [PubMed] [Google Scholar]
- 39.Ramezani A., Aghakhani A., Sharif M.R., et al. Anemia prevalence and related factors in HIV-infected patients: a cohort study. International Journal of Family Psychiatry. 2008;57(3):284–289. [Google Scholar]
- 40.Mildvan D., Creagh T., Leitz G. Prevalence of anemia and correlation with biomarkers and specific antiretroviral regimens in 9690 human-immunodeficiency-virus-infected patients: findings of the anemia prevalence study. Curr Med Res Opin. 2007;23(2):343–355. doi: 10.1185/030079906X162683. [DOI] [PubMed] [Google Scholar]
- 41.Bayleyegn B., Woldu B., Yalew A., et al. Magnitude and associated factors of peripheral cytopenia among HIV-infected children attending at University of Gondar Specialized Referral Hospital, Northwest Ethiopia. PLoS One. 2021;16(3) doi: 10.1371/journal.pone.0247878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melku M., Enawgaw B., Ayana S., et al. Magnitude of anemia and undernutrition amon HIV-infected children who took HAART: a retrospective follow-up study. Am J Blood Res. 2020;10(5):198–209. [PMC free article] [PubMed] [Google Scholar]
- 43.Tsegay Y.G., Tadele A., Addis Z., et al. Magnitude of cytopenias among HIV-infected children in Bahir Dar, northwest Ethiopia: a comparison of HAART-naïve and HAART-experienced children. HIV AIDS (Auckl) 2017;9:31–42. doi: 10.2147/HIV.S125958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shet A., Bhavani P.K., Kumarasamy N., et al. Anemia, diet and therapeutic iron among children living with HIV: a prospective cohort study. BMC Pediatr. 2015;15:164. doi: 10.1186/s12887-015-0484-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ezeonwu B.U., Ikefuna A.N., Oguonu T., et al. Prevalence of hematological abnormalities and malnutrition in HIV-infected under five children in Enugu. Niger J Clin Pract. 2014;17(3):303–308. doi: 10.4103/1119-3077.130230. [DOI] [PubMed] [Google Scholar]
- 46.Kapavarapu P.K., Bari O., Perumpil M., et al. Growth patterns and anaemia status of HIV-infected children living in an institutional facility in India. Trop Med Int Health. 2012;17(8):962–971. doi: 10.1111/j.1365-3156.2012.03022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shet A., Arumugam K., Rajagopalan N., et al. The prevalence and etiology of anemia among HIV-infected children in India. Eur J Pediatr. 2012;171(3):531–540. doi: 10.1007/s00431-011-1599-y. [DOI] [PubMed] [Google Scholar]
- 48.Damtie S., Workineh L., Kiros T., et al. Hematological abnormalities of adult HIV-infected patients before and after initiation of highly active antiretroviral treatment at debre tabor comprehensive specialized hospital, northcentral ethiopia: a cross-sectional study. HIV AIDS (Auckl) 2021;13:477–484. doi: 10.2147/HIV.S308422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Butt N.I., Tehseen N., Malik T., et al. Is anaemia frequent in HIV/AIDS patients presenting to a tertiary care hospital? J Pak Med Assoc. 2020;70(12–B):2368–2370. doi: 10.47391/JPMA.321. [DOI] [PubMed] [Google Scholar]
- 50.Baye M., Fisseha B., Bayisa M., et al. Experience of fatigue and associated factors among adult people living with HIV attending ART clinic: a hospital-based cross-sectional study in Ethiopia. BMJ Open. 2020;10(10) doi: 10.1136/bmjopen-2020-042029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berhane Y., Haile D., Tolessa T. Anemia in HIV/AIDS patients on antiretroviral treatment at Ayder specialized hospital, Mekele, Ethiopia: a case-control study. J Blood Med. 2020;11:379–387. doi: 10.2147/JBM.S275467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khatri S., Amatya A., Shrestha B. Nutritional status and the associated factors among people living with HIV: an evidence from cross-sectional survey in hospital based antiretroviral therapy site in Kathmandu, Nepal. BMC Nutr. 2020;6:22. doi: 10.1186/s40795-020-00346-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sah S.K., Dahal P., Tamang G.B., et al. Prevalence and predictors of anemia in HIV-infected persons in Nepal. HIV AIDS (Auckl) 2020;12:193–200. doi: 10.2147/HIV.S244618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zanni M.V., Currier J.S., Kantor A., et al. Correlates and timing of reproductive aging transitions in a global cohort of midlife women with human immunodeficiency virus: insights from the REPRIEVE trial. J Infect Dis. 2020;222:S20–S30. doi: 10.1093/infdis/jiaa214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ageru T.A., Koyra M.M., Gidebo K.D., et al. Anemia and its associated factors among adult people living with human immunodeficiency virus at Wolaita Sodo University teaching referral hospital. PLoS One. 2019;14(10):e0221853. doi: 10.1371/journal.pone.0221853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tamir Z., Seid A., Haileslassie H. Magnitude and associated factors of cytopenias among antiretroviral therapy naive Human Immunodeficiency virus infected adults in Dessie, Northeast Ethiopia. PLoS One. 2019;14(2):e0211708. doi: 10.1371/journal.pone.0211708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yesuf T., Muhie O.A., Shibru H. Prevalence and predictors of anemia among adult HIV infected patients at the University of Gondar Hospital, Northwest Ethiopia. HIV AIDS (Auckl) 2019;11:211–217. doi: 10.2147/HIV.S209446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ezeamama A.E., Guwatudde D., Sikorskii A., et al. Impaired hematologic status in relation to clinical outcomes among HIV-infected adults from Uganda: a prospective cohort study. Nutrients. 2018;10(4):475. doi: 10.3390/nu10040475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hentzien M., Delpierre C., Pugliese P., et al. Derivation and internal validation of a mortality risk index for aged people living with HIV: the Dat'AIDS score. PLoS One. 2018;13(4) doi: 10.1371/journal.pone.0195725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Katemba C., Muzoora C., Muwanguzi E., et al. Hematological abnormalities in HIV-antiretroviral therapy naïve clients as seen at an immune suppression syndrome clinic at Mbarara regional referral hospital, southwestern Uganda. J Blood Med. 2018;9:105–110. doi: 10.2147/JBM.S157148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beyene H.B., Tadesse M., Disassa H., et al. Concurrent Plasmodium infection, anemia and their correlates among newly diagnosed people living with HIV/AIDS in Northern Ethiopia. Acta Trop. 2017;169:8–13. doi: 10.1016/j.actatropica.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 62.Fiseha T., Tamir Z., Seid A., et al. Prevalence of anemia in renal insufficiency among HIV infected patients initiating ART at a hospital in Northeast Ethiopia. BMC Hematol. 2017;17(1) doi: 10.1186/s12878-017-0071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jin Y., Li Q., Meng X., et al. Prevalence of anaemia among HIV patients in rural China during the HAART era. Int J STD AIDS. 2017;28(1):63–68. doi: 10.1177/0956462415622866. [DOI] [PubMed] [Google Scholar]
- 64.Jin Y., Li Z., Yuan J., et al. Effects of TCM comprehensive intervention on prevalence of anemia among HIV/AIDS patients. China J Tradit Chin Med Pharm. 2017;32(10):4737–4740. [Google Scholar]
- 65.Gunda D.W., Nkandala I., Kilonzo S.B., et al. Prevalence and risk factors of mortality among adult HIV patients initiating ART in rural setting of HIV care and treatment services in North Western Tanzania: a retrospective cohort study. J Sex Transm Dis. 2017;2017 doi: 10.1155/2017/7075601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gunda D.W., Godfrey K.G., Kilonzo S.B., et al. Cytopenias among ART-naive patients with advanced HIV disease on enrolment to care and treatment services at a tertiary hospital in Tanzania: a crosssectional study. Malawi Med J. 2017;29(1):43–52. doi: 10.4314/mmj.v29i1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Melese H., Wassie M.M., Woldie H., et al. Anemia among adult HIV patients in Ethiopia: a hospital-based cross-sectional study. HIV AIDS (Auckl) 2017;9:25–30. doi: 10.2147/HIV.S121021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sahle T., Yemane T., Gedefaw L. Effect of malaria infection on hematological profiles of people living with human immunodeficiency virus in Gambella, southwest Ethiopia. BMC Hematol. 2017;17:2. doi: 10.1186/s12878-017-0072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Widiyanti M., Ubra R., Fitriana E. Low body mass index increases risk of anemia in adults with HIV-AIDS receiving antiretroviral therapy. Universa Med. 2017;36(3):221–227. [Google Scholar]
- 70.Gunda D.W., Kilonzo S.B., Mpondo B.C. Magnitude and correlates of moderate to severe anemia among adult HIV patients receiving first line HAART in Northwestern Tanzania: a cross sectional clinic based study. Pan Afr Med J. 2016;23:26. doi: 10.11604/pamj.2016.23.26.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Obirikorang C., Issahaku R.G., Osakunor D.N., et al. Anaemia and iron homeostasis in a cohort of HIV-infected patients: a cross-sectional study in Ghana. AIDS Res Treat. 2016;2016 doi: 10.1155/2016/1623094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Akinyemi J.O., Adesina O.A., Kuti M.O., et al. Temporal distribution of baseline characteristics and association with early mortality among HIV-positive patients at University College Hospital, Ibadan, Nigeria. Afr J AIDS Res. 2015;14(3):201–207. doi: 10.2989/16085906.2015.1052526. [DOI] [PubMed] [Google Scholar]
- 73.Akilimali P.Z., Kashala-Abotnes E., Musumari P.M., et al. Predictors of persistent anaemia in the first year of antiretroviral therapy: a retrospective cohort study from Goma, the Democratic Republic of Congo. PLoS One. 2015;10(10):e0140240. doi: 10.1371/journal.pone.0140240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kerkhoff A.D., Wood R., Cobelens F.G., et al. The predictive value of current haemoglobin levels for incident tuberculosis and/or mortality during long-term antiretroviral therapy in South Africa: a cohort study. BMC Med. 2015;13:70. doi: 10.1186/s12916-015-0320-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Minchella P.A., Armitage A.E., Darboe B., et al. Elevated hepcidin is part of a complex relation that links mortality with iron homeostasis and anemia in men and women with HIV infection. J Nutr. 2015;145(6):1194–1201. doi: 10.3945/jn.114.203158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mijiti P., Yuexin Z., Min L., et al. Prevalence and predictors of anaemia in patients with HIV infection at the initiation of combined antiretroviral therapy in Xinjiang, China. Int J STD AIDS. 2015;26(3):156–164. doi: 10.1177/0956462414531935. [DOI] [PubMed] [Google Scholar]
- 77.Erqou S., Mohanty A., Murtaza Kasi P., et al. Predictors of mortality among united states veterans with human immunodeficiency virus and hepatitis C virus coinfection. ISRN Gastroenterol. 2014;2014:764540. doi: 10.1155/2014/764540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kerkhoff A.D., Wood R., Cobelens F.G., et al. Resolution of anaemia in a cohort of HIV-infected patients with a high prevalence and incidence of tuberculosis receiving antiretroviral therapy in South Africa. BMC Infect Dis. 2014;14:3860. doi: 10.1186/s12879-014-0702-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kyeyune R., Saathoff E., Ezeamama A.E., et al. Prevalence and correlates of cytopenias in HIV-infected adults initiating highly active antiretroviral therapy in Uganda. BMC Infect Dis. 2014;14:496. doi: 10.1186/1471-2334-14-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martin C., Poudel-Tandukar K., Poudel K.C. HIV symptom burden and anemia among HIV-positive individuals: cross-sectional results of a community-based positive living with HIV (POLH) study in Nepal. PLoS One. 2014;9(12) doi: 10.1371/journal.pone.0116263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Santiago-Rodriguez E.J., Mayor A.M., Fernandez-Santos D.M., et al. Anemia in a cohort of HIV-infected Hispanics: prevalence, associated factors and impact on one-year mortality. BMC Res Notes. 2014;7:439. doi: 10.1186/1756-0500-7-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shet A., Antony J., Arumugam K., et al. Influence of adverse drug reactions on treatment success: prospective cohort analysis of HIV-infected individuals initiating first-line antiretroviral therapy in India. PLoS One. 2014;9(3):e91028. doi: 10.1371/journal.pone.0091028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tesfaye Z., Enawgaw B. Prevalence of anemia before and after initiation of highly active antiretroviral therapy among HIV positive patients in Northwest Ethiopia: a retrospective study. BMC Res Notes. 2014;7:745. doi: 10.1186/1756-0500-7-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ferede G., Wondimeneh Y. Prevalence and related factors of anemia in HAART-naive HIV positive patients at Gondar University Hospital, Northwest Ethiopia. BMC Hematol. 2013;13(1):8. doi: 10.1186/2052-1839-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hadgu T.H., Worku W., Tetemke D., et al. Undernutrition among HIV positive women in Humera hospital, Tigray, Ethiopia, 2013: antiretroviral therapy alone is not enough, cross sectional study. BMC Public Health. 2013;13:943. doi: 10.1186/1471-2458-13-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Khasanova G.R., Bikkinina O.I., Anokhin V.A. Depression in young HIV-positive patients is associated with anemia. Kazanskiĭ Meditsinskiĭ Zhurnal. 2013;94(4):445–450. [Google Scholar]
- 87.Liu Z., Jin Y., Yang J., et al. Proceedings of the IEEE International Conference on Bioinformatics and Biomedicine. 2013. Prevalence and related factors of anemia among human immunodeficiency virus(HIV)/acquired immune deficiency syndrome (AIDS) outpatients in resources limited region of China. In: Li GZ, Kim S, Hughes M, et al., eds. [Google Scholar]
- 88.Zhou J., Jaquet A., Bissagnene E., et al. Short-term risk of anaemia following initiation of combination antiretroviral treatment in HIV-infected patients in countries in sub-Saharan Africa, Asia-Pacific, and central and South America. J Int AIDS Soc. 2012;15 doi: 10.1186/1758-2652-15-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Akinbo F.O., Omoregie R. Plasmodium falciparum infection in HIV-infected patients on highly active antiretroviral therapy (HAART) in Benin City, Nigeria. J Res Health Sci. 2012;12(1):15–18. [PubMed] [Google Scholar]
- 90.Meidani M., Rezaei F., Maracy M.R., et al. Prevalence, severity, and related factors of anemia in HIV/AIDS patients. J Res Med Sci. 2012;17(2):138–142. [PMC free article] [PubMed] [Google Scholar]
- 91.Subbaraman R., Devaleenal B., Selvamuthu P., et al. Factors associated with anaemia in HIV-infected individuals in southern India. Int J STD AIDS. 2009;20(7):489–492. doi: 10.1258/ijsa.2008.008370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Erhabor O., Babatunde S., Uko K.E. Some haematological parameters in plasmodial parasitized HIV-infected Nigerians. Niger J Med. 2006;15(1):52–55. doi: 10.4314/njm.v15i1.37116. [DOI] [PubMed] [Google Scholar]
- 93.Berhane K., Karim R., Cohen M.H., et al. Impact of highly active antiretroviral therapy on anemia and relationship between anemia and survival in a large cohort of HIV-infected women - Women's interagency HIV study. JAIDS J Acquir Immune Defic Syndr. 2004;37(2):1245–1252. doi: 10.1097/01.qai.0000134759.01684.27. [DOI] [PubMed] [Google Scholar]
- 94.Semba R.D., Shah N., Klein R.S., et al. Highly active antiretroviral therapy associated with improved anemia among HIV-infected women. AIDS Patient Care STDs. 2001;15(9):473–480. doi: 10.1089/108729101753145466. [DOI] [PubMed] [Google Scholar]
- 95.Delicio A.M., Lajos G.J., Amaral E., et al. Adverse effects of antiretroviral therapy in pregnant women infected with HIV in Brazil from 2000 to 2015: a cohort study. BMC Infect Dis. 2018;18(1):485. doi: 10.1186/s12879-018-3397-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Manyanga V.P., Minzi O., Ngasala B. Prevalence of malaria and anaemia among HIV infected pregnant women receiving co-trimoxazole prophylaxis in Tanzania: a cross sectional study in Kinondoni Municipality. BMC Pharmacol Toxicol. 2014;15:24. doi: 10.1186/2050-6511-15-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ezechi O.C., Kalejaiye O.O., Gab-Okafor C.V., et al. The burden of anaemia and associated factors in HIV positive Nigerian women. Arch Gynecol Obstet. 2013;287(2):239–244. doi: 10.1007/s00404-012-2573-2. [DOI] [PubMed] [Google Scholar]
- 98.Calis J.C., van Hensbroek M.B., de Haan R.J., et al. HIV-associated anemia in children: a systematic review from a global perspective. AIDS. 2008;22(10):1099–1112. doi: 10.1097/QAD.0b013e3282fa759f. [DOI] [PubMed] [Google Scholar]
- 99.Abioye A.I., Andersen C.T., Sudfeld C.R., et al. Anemia, iron status, and HIV: a systematic review of the evidence. Adv Nutr. 2020;11(5):1334–1363. doi: 10.1093/advances/nmaa037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kreuzer K.A., Rockstroh J.K. Pathogenesis and pathophysiology of anemia in HIV infection. Ann Hematol. 1997;75(5–6):179–187. doi: 10.1007/s002770050340. [DOI] [PubMed] [Google Scholar]
- 101.Scadden D.T., Zeira M., Woon A., et al. Human immunodeficiency virus infection of human bone marrow stromal fibroblasts. Blood. 1990;76(2):317–322. [PubMed] [Google Scholar]
- 102.Ageru T.A., Koyra M.M., Gidebo K.D., et al. Anemia and its associated factors among adult people living with human immunodeficiency virus at Wolaita Sodo University teaching referral hospital. PLoS One. 2019;14(10) doi: 10.1371/journal.pone.0221853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cox C.M., El-Mallawany N.K., Kabue M., et al. Clinical characteristics and outcomes of HIV-infected children diagnosed with kaposi sarcoma in malawi and botswana. Pediatr Blood Cancer. 2013;60(8):1274–1280. doi: 10.1002/pbc.24516. [DOI] [PubMed] [Google Scholar]
- 104.Kerkhoff A.D., Meintjes G., Opie J., et al. Anaemia in patients with HIV-associated TB: relative contributions of anaemia of chronic disease and iron deficiency. Int J Tuberc Lung Dis. 2016;20(2):193–201. doi: 10.5588/ijtld.15.0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dai G., Xiao J., Gao G., et al. Anemia in combined antiretroviral treatment-naive HIV-infected patients in China: a retrospective study of prevalence, risk factors, and mortality. Biosci Trends. 2016;10(6):445–453. doi: 10.5582/bst.2016.01165. [DOI] [PubMed] [Google Scholar]
- 106.Enawgaw B., Alem M., Addis Z., et al. Determination of hematological and immunological parameters among HIV positive patients taking highly active antiretroviral treatment and treatment naive in the antiretroviral therapy clinic of Gondar University Hospital, Gondar, Northwest Ethiopia: a comparative cross-sectional study. BMC Hematol. 2014;14(1):8. doi: 10.1186/2052-1839-14-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chet L., Hamid S., Bachok N., et al. Survival and prognostic factors of HIV-positive patients after antiretroviral therapy initiation at a Malaysian referral hospital. Saudi J Med Med Sci. 2021;9(2):135–144. doi: 10.4103/sjmms.sjmms_72_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Peierdun M.J., Liu W.X., Renaguli A.Z., et al. Clinical characteristics of abnormal savda syndrome type in human immunodeficiency virus infection and acquired immune deficiency syndrome patients: a cross-sectional investigation in Xinjiang, China. Chin J Integr Med. 2015;21(12):895–901. doi: 10.1007/s11655-015-2075-8. [DOI] [PubMed] [Google Scholar]
- 109.Owiredu W.K., Quaye L., Amidu N., et al. Prevalence of anaemia and immunological markers among ghanaian HAART-naïve HIV-patients and those on HAART. Afr Health Sci. 2011;11(1):2–15. [PMC free article] [PubMed] [Google Scholar]
- 110.Tay S.C., Badu K., Mensah A.A., et al. The prevalence of malaria among HIV seropositive individuals and the impact of the co- infection on their hemoglobin levels. Ann Clin Microbiol Antimicrob. 2015;14:10. doi: 10.1186/s12941-015-0064-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Denue B.A., Gashau W., Bello H.S., et al. Relation between some haematological abnormalities, degree of immunosuppression and viral load in treatment-naive HIV-infected patients. East Mediterr Health J. 2013;19(4):362–368. [PubMed] [Google Scholar]
- 112.Kiragga A.N., Castelnuovo B., Nakanjako D., et al. Baseline severe anaemia should not preclude use of zidovudine in antiretroviral-eligible patients in resource-limited settings. J Int AIDS Soc. 2010;13 doi: 10.1186/1758-2652-13-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Firnhaber C., Smeaton L., Saukila N., et al. Comparisons of anemia, thrombocytopenia, and neutropenia at initiation of HIV antiretroviral therapy in Africa, Asia, and the Americas. Int J Infect Dis. 2010;14(12):e1088–e1092. doi: 10.1016/j.ijid.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mocroft A., Kirk O., Barton S.E., et al. Anaemia is an independent predictive marker for clinical prognosis in HIV-infected patients from across Europe. EuroSIDA study group. AIDS. 1999;13(8):943–950. doi: 10.1097/00002030-199905280-00010. [DOI] [PubMed] [Google Scholar]
- 115.Phe T., Thai S., Veng C., et al. Risk factors of treatment-limiting anemia after substitution of zidovudine for stavudine in HIV-infected adult patients on antiretroviral treatment. PLoS One. 2013;8(3):e60206. doi: 10.1371/journal.pone.0060206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhou J., Jaquet A., Bissagnene E., et al. Short-term risk of anaemia following initiation of combination antiretroviral treatment in HIV-infected patients in countries in sub-Saharan Africa, Asia-Pacific, and central and South America. J Int AIDS Soc. 2012;15(1):5. doi: 10.1186/1758-2652-15-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Reust C.E. Common adverse effects of antiretroviral therapy for HIV disease. Am Fam Phys. 2011;83(12):1443–1451. [PubMed] [Google Scholar]
- 118.Sullivan P. Associations of anemia, treatments for anemia, and survival in patients with human immunodeficiency virus infection. J Infect Dis. 2002;185 (Suppl 2):S138–S142. doi: 10.1086/340203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.