Fig. 4.
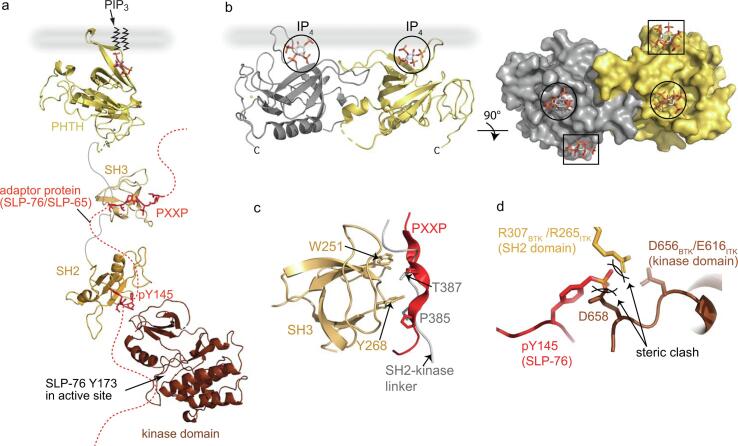
Activation of the TEC kinases. T-cell or B-cell stimulation generates ligands for the regulatory domains of the TEC kinases which promote the formation of signaling complexes that nucleate around non-catalytic scaffolding proteins such as SLP-76 in T-cells (or SLP-65 in B cells). These interactions with the regulatory domains disrupt the ‘closed’ autoinhibited conformation of TEC kinases to a more ‘open’ form (a). Crystal structures reveal dimerization (b, left) of the BTK N-terminal PHTH domain bound to the PIP3 headgroup, IP4, now known as the ‘Saraste dimer’. Subsequent structures of the BTK PHTH dimer (Wang et al., 2015) revealed additional phospholipid binding sites; the canonical site (circled) as well as a peripheral site (boxed) on each domain in the dimer (b, right). Elegant studies using fluorescence correlation spectroscopy and membrane-binding kinetic measurements revealed a role for the peripheral site in setting a PIP3 threshold required to trigger BTK activation (Chung et al., 2019). (c) Interactions between the PXXP motif (red) in adaptor proteins and the SH3 domains of ITK/BTK (Bunnell et al., 2000) contribute to the unraveling of the compact autoinhibitory pose by displacing the SH2-kinase linker (grey). (d) Phosphotyrosine binding (SLP-76 pY145 is shown in (a)) to the ITK/BTK SH2 domain is sterically incompatible with the autoinhibitory interaction between the SH2 and the C-terminal region of the kinase domain (shown in Fig. 2a,iii) leading to displacement of the intramolecular SH2 inhibitory interaction and a shift toward the active conformation. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)