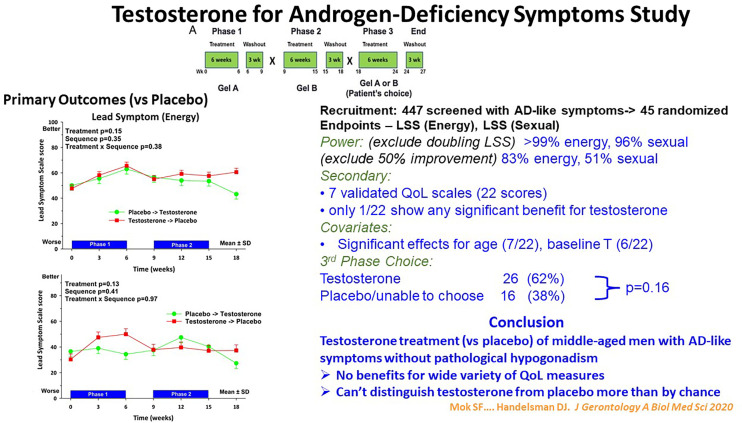
Figure 3.
Summary of the Testosterone for Androgen-Deficiency Symptoms (TADS) Study, a randomized placebo-controlled cross-over clinical trial with a unique third, masked choice extension phase for 45 men with androgen deficiency-type symptoms in energy and sexual domains but without pathologic hypogonadism. In the third phase the participants, remaining blinded to their original treatment, chose which of the two previous treatment they regarded as better for the third phase. Despite adequate power, the participants reported no significant benefit of testosterone treatment nor could distinguish it from placebo more than by chance. For details see Mok et al. (66).