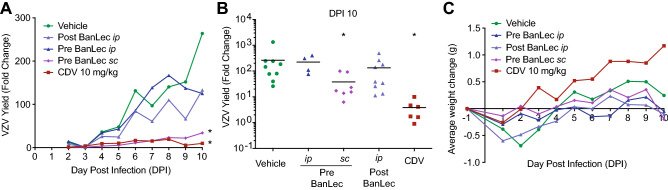
Figure 7.
H84T BanLec evaluation in NuSkin mice infected with VZV. NuSkin mice were implanted with a single subcutaneous xenograft of adult human skin and xenografts were inoculated 3–4 weeks later with VZV by intraxenograft injection and scarification. Mice were treated with 50 mg/kg/day H84T BanLec by subcutaneous (sc) or intraperitoneal (ip) routes given 6 h pre-inoculation (−6 HPI) and on DPI 4 and 8 to the Pre-BanLec groups, or by the ip route on DPI 3 and 7 to the Post-BanLec group. Cidofovir was administered daily by ip injection from DPI 3–9. (A) VZV yield was measured by bioluminescence and the fold change calculated as the Total Flux on each day divided by the Total Flux on DPI 2 or 3 (the lowest value for that mouse). Lines represent the average VZV yield per group. (B) VZV yield on DPI 10 was analyzed for statistical significance where each symbol represents one mouse, and the bar is the mean of the group. (*) Signifies statistical significance between the vehicle and a treatment group (p < 0.05, one-way ANOVA, Dunnett’s post hoc test). (C) Mouse weights were measured daily, and the lines show the average weight change for each group from the onset to conclusion of the study. N = 4–12 mice per group.