Abstract
Background and Objectives:
Despite the broad utilization of component-based transfusion strategies that aim to reconstitute whole blood during acute traumatic hemorrhage, data for hemorrhage occurring outside of trauma and surgery are limited.
Methods:
This is an observational cohort study of adults experiencing critical non-traumatic, non-intraoperative hemorrhage during hospitalization at an academic medical center from 2011-2015. The primary goal was to evaluate differences in plasma and platelet to red blood cell (RBC) transfusion ratios across patient demographic, clinical, and laboratory characteristics. Secondarily, associations between transfusion ratios and clinical outcomes were assessed.
Results:
709 patients were included: 498 (70.2%) medical and 211 (29.8%) post-surgical. The gastrointestinal tract (36.7%) was the most common site of bleeding. Most patients received RBCs without plasma (35.5%) or platelets (54.2%). Among those receiving plasma, 82.3% received a plasma to RBC ratio <1:1 at 24 hours. For platelets, the most common ratio was 1-2:1 (52.9%). Transfusion ratios were generally consistent across comorbid disease severity, admission type, and anatomic sites of bleeding. Higher plasma utilization was observed in the emergency department, while greater platelet utilization occurred in intensive care units. Higher transfusion ratios were observed in those with greater laboratory hemostatic abnormalities prior to the hemorrhagic event. Clinical outcome differences were limited, though greater platelet utilization in the first 24 hours was associated with higher mortality and fewer hospital free days.
Conclusions:
Transfusion ratios for critical non-traumatic hemorrhage were primarily related to laboratory abnormalities preceding the hemorrhagic event and practice environments. Clinical outcome differences across ratios were limited.
Keywords: Transfusion ratios, critical administration threshold, hemorrhage
Introduction
Transfusion of plasma, platelets, and red blood cells (RBCs) in relatively fixed high ratios (i.e. 1:1:1) as part of a balanced resuscitation strategy has become a standard of care for patients suffering acute traumatic hemorrhage.[1–4] Extrapolating from the trauma experience, most institutions adopted “massive transfusion” protocols with empiric high ratio strategies to facilitate standardized blood component delivery in times of acute exsanguination, irrespective of any perceived relationship to traumatic injury.[5,6]
Most massive transfusion events occur in patients without trauma, yet the safety and efficacy of extrapolation of trauma-based resuscitation strategies to non-trauma populations remains unclear.[7–10] While recent observational data suggests that higher transfusion ratios may not be associated with improved outcomes in surgical patients with non-traumatic intraoperative hemorrhage,[11] evidence for optimal transfusion ratios outside of operative and acute trauma resuscitation settings is limited. Patients that suffer life-threatening hemorrhage secondary to non-traumatic insults are likely to be phenotypically and physiologically distinct from their trauma counterparts. Hence, it is important to assess the application of ratio-based transfusions outside of trauma, with a focus on clinical and environmental factors that may lead to differences in resuscitation strategies and clinical outcomes.
The primary goal of this study is to assess plasma, platelet, and RBC transfusion strategies in patients with critical non-traumatic and non-intraoperative hemorrhage, with an emphasis on differences in transfusion ratios based upon anatomic sources of bleeding and patient demographic, clinical, and laboratory features. Additionally, we assess the relationships between transfusion ratios and clinical outcomes, which may be utilized for hypothesis generation to inform future clinical trials regarding optimal resuscitation strategies for non-traumatic hemorrhage.
Methods
This is an observational cohort study conducted under approval of the local Institutional Review Board (Mayo Clinic, Rochester, MN) at a single academic medical center with waived requirement for written informed consent, though patients previously declining medical record use for observational research were excluded. The study was conducted in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.[12]
Inclusion criteria were hospitalized patients aged 18 years or older experiencing hemorrhage requiring large-volume transfusion according to the critical administration threshold (CAT) during a study period of January 1st, 2011 and December 31st, 2015. The CAT is used to identify patients with rapid life-threatening bleeding requiring large volume transfusion and is defined by transfusion of 3 or more units of RBCs within a 1 hour time period.[13] It has several advantages over other commonly-employed definitions of “massive transfusion” (e.g. ≥ 10 units of RBCs in 24 hours). It reduces the potential impact of survivor bias in observational studies, facilitates earlier identification of patients with life-threatening hemorrhage, and is predictive of mortality.[13–15] Exclusion criteria included: traumatic injury, transfusions administered intraoperatively for surgical patients, and previous denial of authorization of medical record use for observational research. Post-surgical patients meeting CAT criteria who subsequently returned to the operating room due to bleeding were included, though patients with primary intraoperative hemorrhage were not included, as this data has previously been reported.[11] Patients were only included in the study once, such that only the first CAT event was included for any given patient. CAT events were pre-specified to extend 24 hours from the time of the first transfused unit.
A transfusion protocol for critical hemorrhage (i.e. massive transfusion protocol) was implemented at the study institution in 2006, which facilitated emergent release of blood products to all clinical areas. This protocol remained unchanged throughout the study period and was activated by direct communication with the blood bank via phone call, face-to-face dialogue, or electronically. Activation triggered release of 6 units of uncrossmatched O-negative RBCs, 6 units of type A or AB plasma, and 1 unit of Rh-negative apheresis platelets (equivalent to a 6 pack of pooled platelets), with the goal of approximating 1:1:1 whole blood resuscitation. Administration of individual components was at the discretion of the attending clinician.
All adult patients meeting CAT criteria were identified using the Transfusion DataMart, an institutional data warehouse containing comprehensive data surrounding each unit of ordered allogeneic blood, including transfusion timing and associated laboratory values. Clinical features were obtained through the ICU DataMart, another institutional datamart containing detailed information of patient features in acute-care environments, and through the Advanced Cohort Explorer (ACE), which provides a real-time feed of the electronic medical record. These resources are highly-accurate and undergo continuous monitoring and validation, as reported previously.[16,17]
Baseline demographic and clinical characteristics were extracted for all patients, including age, sex, Charlson comorbidity score, past medical history, pre-transfusion laboratory values including hemoglobin, platelet count, and INR (i.e. the most recent values in the 24 hours preceding the first blood product administration during the CAT event), and administration of antiplatelet, antithrombotic, antifibrinolytic, and hemostatic therapies. The physical location [i.e. intensive care unit (ICU), emergency department (ED), or hospital floor] of the first transfused product was also extracted. Manual chart review (LJM, EBK) was utilized to categorize anatomic locations of bleeding. Transfusions of plasma, platelets, and RBCs were extracted throughout hospitalization. Recognizing that transfusion ratios change throughout resuscitation, plasma to RBC and platelet to RBC ratios were calculated at 3, 12, and 24 hours, with appropriate censoring for those dying prior to the interval of interest. Patients meeting massive transfusion criteria by both CAT and traditionally-defined metrics (i.e. ≥10 RBCs within 24 hours) were also identified.
Statistical Considerations:
Data were descriptively summarized using frequency and percent for categorical variables and medians and interquartile ranges (IQR) for continuous variables. Between group comparisons of demographic, clinical, and laboratory variables based on plasma to RBC or platelet to RBC ratios were performed using Chi-square and Fisher exact tests for categorical variables and Kruskal-Wallis tests for continuous variables, respectively. Missing data were handled using multiple imputation with 25 independent imputed datasets. Missing variables included: pre-transfusion INR (18.6%), pre-transfusion platelet count (1.4%), and pre-transfusion hemoglobin (0.2%).
For exploratory analyses of clinical outcomes across transfusion strategies, plasma to RBC ratios were divided into 4 quartiles designed to maximize group size while also encompassing the most commonly utilized and clinically applicable transfusion ratios of 1:1 and 1:2, consistent with previous research.[11] These quartile ratios were: 0 (no plasma), 0.1-0.4 (i.e. ratio >0 but <1:2), 0.5-0.9 (i.e. ratio ≥1:2 but <1:1), and >1 (i.e. ratio ≥1:1). Platelet to RBC ratios were similarly divided into 4 quartiles: 0 (no platelets), 0.1-0.9 (i.e. ratio ≥0 but <1:1), 1.0–2.0 (i.e. ratio ≥1:1 but ≤2:1), and >2 (i.e. ratio >2:1).
Clinical outcomes included all-cause hospital mortality and hospital free days. Free days were calculated as 28 minus the hospital length of stay in days, with patients dying prior to day 28 or those with lengths of stay greater than 28 days receiving a score of 0. The relationships between clinical outcomes and plasma to RBC and platelet to RBC ratios at 3, 12, and 24 hours were analyzed utilizing multivariable regression models adjusted for age, sex, Charlson score, pre-CAT+ labs (hemoglobin, platelet count, INR), antiplatelet therapy, anticoagulants, hemostatic agents, and the transfused volumes of plasma, platelets, and allogeneic RBCs at the time of the outcome assessment interval (i.e. 3, 12, 24 hours). For analyses of plasma to RBC ratios, we additionally adjusted for the corresponding platelet to RBC ratio at the same time interval, and vice versa. The PCC utilized during the study period was a 3-factor PCC (Bebulin®, Shire Plc). Hospital mortality was assessed with multivariable logistic regression, while hospital free days were modeled using linear regression. Predefined sensitivity analyses were performed excluding patients not receiving plasma or platelet therapies in the first 24 hours and limited to those receiving traditional massive transfusion (≥10 units RBCs within 24 hours). A two-tailed p-value < 0.05 was utilized to determine statistical significance without correction for multiple comparisons given the hypothesis-generating nature of secondary clinical outcome analyses.
Results
A total of 709 patients were included (Figure 1): 498 (70.2%) with medical hemorrhage and 211 (29.8%) with post-surgical hemorrhage. The median (IQR) age was 65 (53, 76) years. Most patients were male (58.4%) with median Charlson comorbidity index scores of 6 (4, 9). Most bleeds originated in the gastrointestinal tract (36.7%) followed by intraabdominal (19.3%) and thoracic (12.4%) bleeding (Figure 2).
Figure 1.
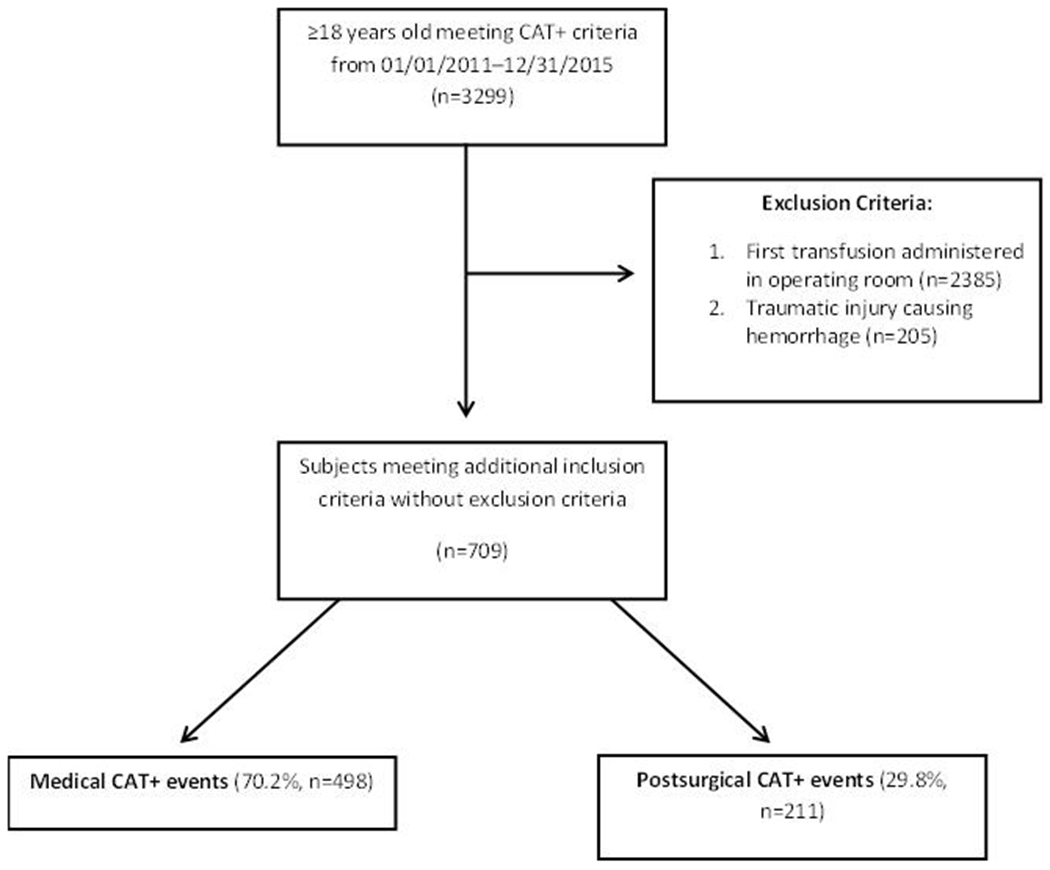
Patient flow diagram
CAT – critical administration threshold
Figure 2.
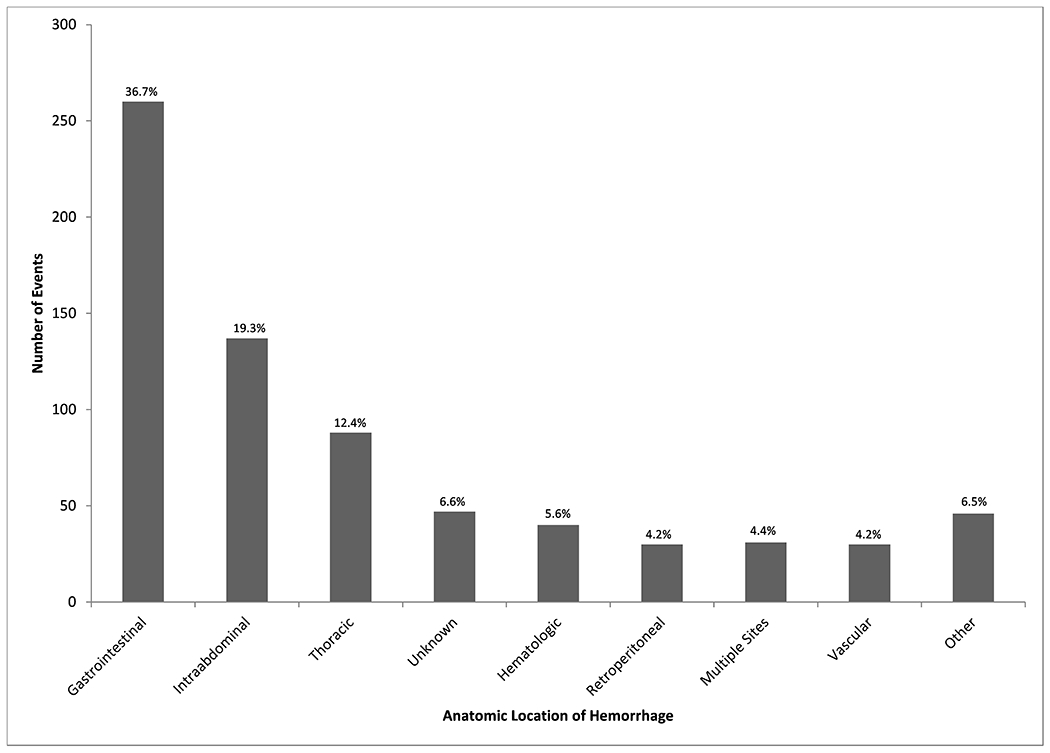
Anatomic location of source of CAT+ hemorrhagic event
Patient demographic and clinical characteristics stratified by plasma to RBC and platelet to RBC ratios are displayed in Tables 1 and 2, respectively. There were no clear differences in transfusion ratios based upon patient sex, Charlson score, admission type (medical vs. post-surgical), or anatomic source of bleeding. Transfusion ratios decreased modestly with increasing age. The median RBC transfusion volume was 5 (4, 7) units, which did not increase uniformly with transfusion ratios. There were significant differences in plasma (p=0.02) and platelet to RBC (p=0.009) ratios based upon the hospital location in which transfusion was initiated such that the proportion of patients transfused in the emergency department (ED) increased with higher plasma to RBC ratios and the proportion of patients transfused in the ICU increased with higher platelet to RBC ratios. As an example, 21.6% (19/88) of patients transfused in the ED achieved a plasma to RBC ratio >1 compared to 10.2% (59/577) of patients first transfused in the ICU. Conversely, 34.8% (201/577) of patients transfused in the ICU achieved a platelet to RBC ratio >1 compared to 19.3% (17/88) in the ED. Abnormalities in hemostatic laboratory tests (i.e. platelet count, INR) prior to the event were more frequently observed with higher ratios. Higher rates of warfarin therapy were observed in patients receiving higher plasma-to-RBC ratios. Aspirin therapy was greatest in those not receiving plasma or platelets, with the lowest rate of therapy in those receiving the highest platelet-to-RBC ratio. Antifibrinolytic therapy was utilized in only 3.8% of cases, with increasing use with higher transfusion ratios. PCCs were administered in less than 2% of cases.
Table 1.
Patient Demographics and Clinical Characteristics By Plasma:RBC ratio at 24 hours
| Characteristic | 0 N=252 |
0.1-0.4 N=172 |
0.5-0.9 N=204 |
1.0+ N=81 |
Total N=709 |
p-value |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age | 66.5 (54.2, 76.9) | 65.4 (55.6, 76.3) | 63.3 (50.9, 74.4) | 61.9 (47.1, 71.9) | 64.6 (52.8, 75.8) | 0.03 † |
| Male Sex | 143 (56.7%) | 104 (60.5%) | 125 (61.3%) | 42 (51.9%) | 414 (58.4%) | 0.44 ‡ |
| Charlson Score | 6 (4, 9) | 6 (4, 9) | 6.0 (3.5, 8.0) | 5 (2, 8) | 6 (4, 9) | 0.07 † |
| Massive Transfusion + | 16 (6.3%) | 52 (30.2%) | 70 (34.3%) | 11 (13.6%) | 149 (21.0%) | <0.001 ‡ |
| Patient Location | 0.02 ‡ | |||||
| ICU | 208 (82.5%) | 146 (84.9%) | 164 (80.4%) | 59 (72.8%) | 577 (81.4%) | |
| Emergency Room | 23 (9.1%) | 17 (9.9%) | 29 (14.2%) | 19 (23.5%) | 88 (12.4%) | |
| Floor | 21 (8.3%) | 9 (5.2%) | 11 (5.4%) | 3 (3.7%) | 44 (6.2%) | |
| Anatomical site | 0.76 ‡ | |||||
| Gastrointestinal | 95 (37.7%) | 59 (34.3%) | 73 (35.8%) | 33 (40.7%) | 260 (36.7%) | |
| Intraabdominal | 42 (16.7%) | 36 (20.9%) | 36 (17.6%) | 23 (28.4%) | 137 (19.3%) | |
| Thoracic | 31 (12.3%) | 21 (12.2%) | 28 (13.7%) | 8 (9.9%) | 88 (12.4%) | |
| Hematologic | 15 (6.0%) | 9 (5.2%) | 11 (5.4%) | 5 (6.2%) | 40 (5.6%) | |
| Multifactorial | 9 (3.6%) | 8 (4.7%) | 13 (6.4%) | 1 (1.2%) | 31 (4.4%) | |
| Retroperitoneal | 12 (4.8%) | 9 (5.2%) | 9 (4.4%) | 0 (0.0%) | 30 (4.2%) | |
| Vascular | 11 (4.4%) | 9 (5.2%) | 7 (3.4%) | 3 (3.7%) | 30 (4.2%) | |
| Other | 16 (6.3%) | 11 (6.4%) | 13 (6.4%) | 6 (7.4%) | 46 (6.5%) | |
| Unknown | 21 (8.3%) | 10 (5.8%) | 14 (6.9%) | 2 (2.5%) | 47 (6.6%) | |
| Medical or Post-Surgical | 0.34 ‡ | |||||
| Medical | 181 (71.8%) | 126 (73.3%) | 140 (68.6%) | 51 (63.0%) | 498 (70.2%) | |
| Post-Surgical | 71 (28.2%) | 46 (26.7%) | 64 (31.4%) | 30 (37.0%) | 211 (29.8%) | |
| PLT:RBC Ratio | <0.001 ‡ | |||||
| 0 | 202 (80.2%) | 81 (47.1%) | 68 (33.3%) | 33 (40.7%) | 384 (54.2%) | |
| 0.1-0.9 | 6 (2.4%) | 37 (21.5%) | 45 (22.1%) | 12 (14.8%) | 100 (14.1%) | |
| 1.0-2.0 | 31 (12.3%) | 46 (26.7%) | 69 (33.8%) | 26 (32.1%) | 172 (24.3%) | |
| 2.1+ | 13 (5.2%) | 8 (4.7%) | 22 (10.8%) | 10 (12.3%) | 53 (7.5%) | |
| RBC Units | 4 (3, 5) | 6 (5, 9) | 6.5 (4.0, 10.0) | 4 (3, 7) | 5 (4, 7) | <0.001 † |
| Laboratory values before CAT+ | ||||||
| Hemoglobin,g/dL | 7.1 (6.0, 8.8) | 7.3 (5.7, 8.7) | 7.7 (6.3, 9.1) | 7.4 (6.5, 10.3) | 7.3 (6.0, 9.0) | 0.07 † |
| Platelet Count, x109/L | 164 (100, 258) | 146 (90, 216) | 135 (81, 195) | 139.5 (80.0, 209.0) | 148 (89, 225) | 0.03 † |
| INR | 1.2 (1.1, 1.4) | 1.4 (1.2, 1.8) | 1.4 (1.2, 2.0) | 1.7 (1.3, 2.4) | 1.3 (1.1, 1.8) | <0.001 † |
| Medications before CAT+ | ||||||
| Heparin | 29 (11.5%) | 17 (9.9%) | 15 (7.4%) | 8 (9.9%) | 69 (9.7%) | 0.53 ‡ |
| Direct thrombin inhibitor | 2 (0.8%) | 2 (1.2%) | 1 (0.5%) | 0 (0.0%) | 5 (0.7%) | 0.74 § |
| Warfarin | 42 (16.7%) | 25 (14.5%) | 29 (14.2%) | 23 (28.4%) | 119 (16.8%) | 0.02 ‡ |
| Aspirin | 126 (50.0%) | 75 (43.6%) | 83 (40.7%) | 24 (29.6%) | 308 (43.4%) | 0.01 ‡ |
| LMW Heparin | 25 (9.9%) | 10 (5.8%) | 11 (5.4%) | 6 (7.4%) | 52 (7.3%) | 0.24 ‡ |
| Clopidogrel within 7 Days | 30 (11.9%) | 13 (7.6%) | 16 (7.8%) | 3 (3.7%) | 62 (8.7%) | 0.10 § |
| Factor Xa | 5 (2.0%) | 1 (0.6%) | 2 (1.0%) | 1 (1.2%) | 9 (1.3%) | 0.61 § |
| Hemostatic medications | ||||||
| Vitamin K | 12 (4.8%) | 28 (16.3%) | 25 (12.3%) | 20 (24.7%) | 85 (12.0%) | <0.001 ‡ |
| Antifibrinolytic agents | 3 (1.2%) | 7 (4.1%) | 9 (4.4%) | 8 (9.9%) | 27 (3.8%) | 0.004 § |
| PCCs | 2 (0.8%) | 5 (2.9%) | 3 (1.5%) | 1 (1.2%) | 11 (1.6%) | 0.38 § |
Numbers indicate N (%) unless otherwise noted.
Kruskal-Wallis
Chi-square
Fisher exact
Massive transfusion + defined as administration of ≥10 units of RBCs within 24 hours.
Abbreviations: RBC, red blood cells; PLT, platelets; INR, international normalized ratio; LMW, low molecular weight heparin; CAT, critical administration threshold; PCC, prothrombin complex concentrate.
Table 2.
Patient Demographics and Clinical Characteristics By Platelet:RBC ratio at 24 hours
| Characteristic | 0 N=384 |
0.1-0.9 N=100 |
1.0-2.0 N=172 |
2.1+ N=53 |
Total N=709 |
p-value |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age | 67.1 (55.4, 76.7) | 61.8 (51.0, 73.4) | 61.9 (50.9, 73.4) | 60.9 (47.1, 70.7) | 64.6 (52.8, 75.8) | 0.003 † |
| Male Sex | 226 (58.9%) | 71 (71.0%) | 90 (52.3%) | 27 (50.9%) | 414 (58.4%) | 0.02 ‡ |
| Charlson Score | 6.0 (3.5, 9.0) | 6 (3, 8) | 6 (3, 9) | 6 (4, 8) | 6 (4, 9) | 0.77 † |
| Massive Transfusion + | 21 (5.5%) | 63 (63.0%) | 54 (31.4%) | 11 (20.8%) | 149 (21.0%) | <0.001 ‡ |
| Patient Location | 0.009 ‡ | |||||
| ICU | 300 (78.1%) | 76 (76.0%) | 150 (87.2%) | 51 (96.2%) | 577 (81.4%) | |
| Emergency Room | 54 (14.1%) | 17 (17.0%) | 16 (9.3%) | 1 (1.9%) | 88 (12.4%) | |
| Floor | 30 (7.8%) | 7 (7.0%) | 6 (3.5%) | 1 (1.9%) | 44 (6.2%) | |
| Anatomical Site | 0.10 ‡ | |||||
| Gastrointestinal | 160 (41.7%) | 37 (37.0%) | 50 (29.1%) | 13 (24.5%) | 260 (36.7%) | |
| Intraabdominal | 71 (18.5%) | 19 (19.0%) | 37 (21.5%) | 10 (18.9%) | 137 (19.3%) | |
| Thoracic | 45 (11.7%) | 13 (13.0%) | 23 (13.4%) | 7 (13.2%) | 88 (12.4%) | |
| Hematologic | 19 (4.9%) | 11 (11.0%) | 7 (4.1%) | 3 (5.7%) | 40 (5.6%) | |
| Multifactorial | 15 (3.9%) | 3 (3.0%) | 11 (6.4%) | 2 (3.8%) | 31 (4.4%) | |
| Retroperitoneal | 15 (3.9%) | 4 (4.0%) | 9 (5.2%) | 2 (3.8%) | 30 (4.2%) | |
| Vascular | 12 (3.1%) | 2 (2.0%) | 9 (5.2%) | 7 (13.2%) | 30 (4.2%) | |
| Other | 25 (6.5%) | 4 (4.0%) | 12 (7.0%) | 5 (9.4%) | 46 (6.5%) | |
| Unknown | 22 (5.7%) | 7 (7.0%) | 14 (8.1%) | 4 (7.5%) | 47 (6.6%) | |
| Medical or Post-Surgical | 0.84 ‡ | |||||
| Medical | 270 (70.3%) | 73 (73.0%) | 120 (69.8%) | 35 (66.0%) | 498 (70.2%) | |
| Post-Surgical | 114 (29.7%) | 27 (27.0%) | 52 (30.2%) | 18 (34.0%) | 211 (29.8%) | |
| Plasma:RBC Ratio | <0.001 ‡ | |||||
| 0 | 202 (52.6%) | 6 (6.0%) | 31 (18.0%) | 13 (24.5%) | 252 (35.5%) | |
| 0.1-0.4 | 81 (21.1%) | 37 (37.0%) | 46 (26.7%) | 8 (15.1%) | 172 (24.3%) | |
| 0.5-0.9 | 68 (17.7%) | 45 (45.0%) | 69 (40.1%) | 22 (41.5%) | 204 (28.8%) | |
| 1.0+ | 33 (8.6%) | 12 (12.0%) | 26 (15.1%) | 10 (18.9%) | 81 (11.4%) | |
| RBC Units | 4 (3, 6) | 10 (8, 14) | 6 (4, 9) | 5 (4, 6) | 5 (4, 7) | <0.001 † |
| Laboratory values before CAT+ | ||||||
| Hemoglobin, g/dL | 7.3 (6.0, 9.1) | 7.7 (6.3, 9.1) | 7.3 (6.1, 9.0) | 6.8 (6.0, 8.0) | 7.3 (6.0, 9.0) | 0.14 † |
| Platelet Count, x109/L | 181 (128, 269) | 159 (105, 254) | 104 (60, 146) | 43 (29, 69) | 148 (89, 225) | <0.001 † |
| INR | 1.3 (1.1, 1.7) | 1.3 (1.2, 1.8) | 1.4 (1.2, 1.8) | 1.5 (1.3, 1.9) | 1.3 (1.1, 1.8) | 0.02 † |
| Medications before CAT+ | ||||||
| Heparin | 37 (9.6%) | 10 (10.0%) | 18 (10.5%) | 4 (7.5%) | 69 (9.7%) | 0.94 § |
| Direct thrombin inhibitor | 5 (1.3%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 5 (0.7%) | 0.23 ‡ |
| Warfarin | 83 (21.6%) | 10 (10.0%) | 19 (11.0%) | 7 (13.2%) | 119 (16.8%) | 0.003 ‡ |
| Aspirin | 188 (49.0%) | 36 (36.0%) | 67 (39.0%) | 17 (32.1%) | 308 (43.4%) | 0.01 ‡ |
| Clopidogrel | 36 (9.4%) | 10 (10.0%) | 11 (6.4%) | 5 (9.4%) | 62 (8.7%) | 0.66 ‡ |
| LMW Heparin | 36 (9.4%) | 4 (4.0%) | 10 (5.8%) | 2 (3.8%) | 52 (7.3%) | 0.14 § |
| Factor Xa | 6 (1.6%) | 0 (0.0%) | 2 (1.2%) | 1 (1.9%) | 9 (1.3%) | 0.63 § |
| Hemostatic medications | ||||||
| Vitamin K | 40 (10.4%) | 13 (13.0%) | 22 (12.8%) | 10 (18.9%) | 85 (12.0%) | 0.32 ‡ |
| Antifibrinolytic agents | 11 (2.9%) | 3 (3.0%) | 9 (5.2%) | 4 (7.5%) | 27 (3.8%) | 0.25 § |
| PCCs | 3 (0.8%) | 1 (1.0%) | 7 (4.1%) | 0 (0.0%) | 11 (1.6%) | 0.02 § |
Numbers indicate N (%) unless otherwise noted.
Kruskal-Wallis
Chi-square
Fisher exact
Massive transfusion + defined as administration of ≥10 units of RBCs within 24 hours.
Abbreviations: RBC, red blood cells; INR, international normalized ratio; LMW, low molecular weight heparin; CAT, critical administration threshold; PCC, prothrombin complex concentrate
Patients commonly received RBCs without plasma therapy (35.5%) with median RBC totals of 4 (3, 5) units. Of those receiving plasma, the most common plasma to RBC ratio interval was 0.5-0.9 (44.6%) followed by 0.1-0.4 (37.6%) and ≥ 1 (17.7%). Unadjusted mortality rates by plasma to RBC ratios at 24 hours were 16.1%, 13.4%, 19.0%, and 21.8% for ratios of 0, 0.1-0.4, 0.5-0.9, and > 1, respectively (Figure 3, p=0.38). In multivariable regression models, hospital mortality and free days were not associated with plasma to RBC ratios (Table S1).
Figure 3.
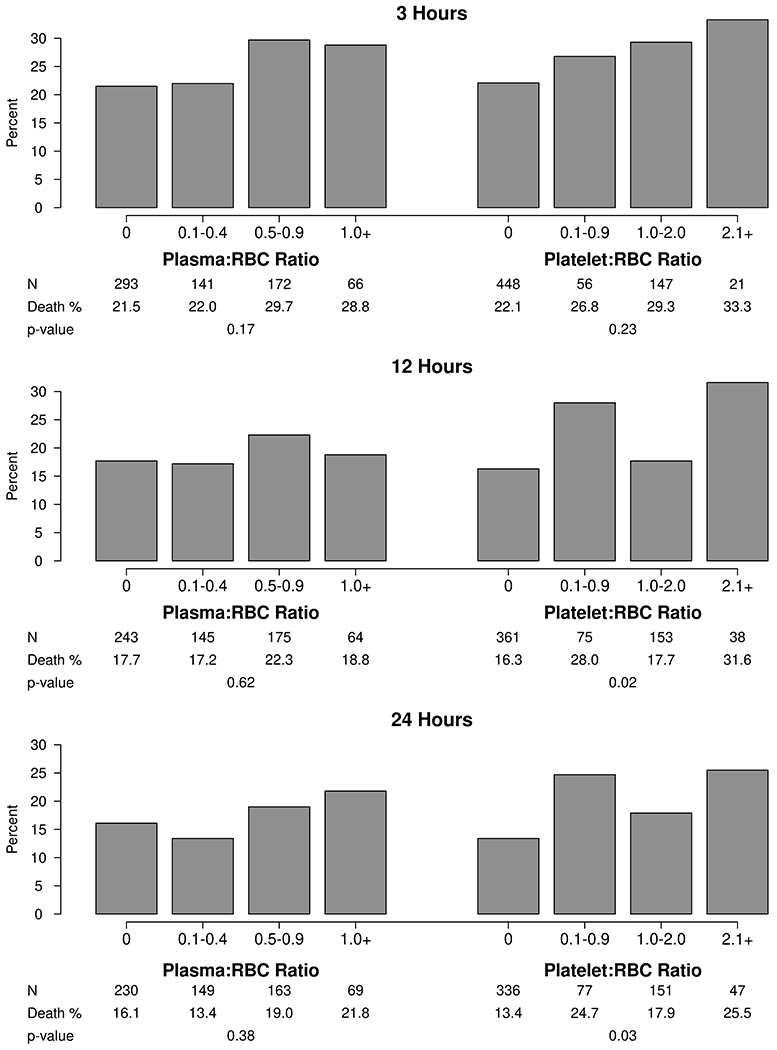
Unadjusted mortality based on plasma to RBC and platelet to RBC ratios at 3, 12, and 24 hours
Similarly, patients often received RBCs without platelets (54.2%) with median RBC totals of 4 (3, 6) units. Of those receiving platelets, the most common platelet to RBC ratio interval was 1-2 (52.9%) followed by 0.1-0.9 (30.8%), and >2 (16.3%). Unadjusted mortality rates by platelet to RBC ratios at 24 hours were 13.4%, 24.7%, 17.9%, and 25.5% for ratios of 0, 0.1-0.9, 1-2, and > 2 (Figure 3, p=0.03). In multivariable analyses (Table S2), a platelet to RBC ratio of 0.1-0.9 at 24 hours was associated with increased hospital mortality [OR (95% CI) 2.2 (1.0, 4.8); p=0.04] and decreased hospital free days [mean (95% CI) decrease 3.2 (0.4, 6.0) days; p=0.02; reference no platelets]. Patients with platelet to RBC ratios > 2 at 12 and 24 hours also had decreased hospital free days. Outcomes were consistent in predefined sensitivity analyses excluding patients not receiving plasma or platelets and limited to those receiving massive transfusion (Tables S3, S4).
Discussion
In this investigation of critical non-traumatic, non-intraoperative hemorrhage, transfusion ratios were generally consistent across patient sex and comorbidity burden but increased concordantly with hemostatic laboratory derangements. There were differences in transfusion strategies based upon the practice environments in which transfusion was initiated but not by anatomical sites of bleeding. Our findings suggest that transfusion strategies for critical non-traumatic hemorrhage are predominantly tailored to laboratory characteristics and clinical practice features. Clinical outcome differences across transfusion strategies were generally limited.
The primary goal of this investigation was to investigate patient and clinical features that may influence transfusion strategies during critical non-traumatic hemorrhage in a large inpatient medical practice. To this end, there were limited differences in transfusion utilization across patient comorbidity burden, sex, admission type (i.e. medical vs. post-surgical), and hemorrhage types, suggesting that providers are less inclined to consider these features when resuscitating abrupt hemorrhage. Additionally, patient age differed only modestly across transfusion ratios, such that patients with advancing age received slightly lower ratios of plasma and platelets to RBCs compared to their younger counterparts. However, there were more obvious differences across transfusion ratio groups in hemostatic laboratory abnormalities, such that patients having more severe derangements in INR and platelet counts received higher ratios. This suggests that clinicians use available laboratory data to drive plasma and platelet component utilization. Further, warfarin use was associated with higher plasma-to-RBC ratios, likely related to higher INR values in those receiving warfarin at the time of hemorrhage, but aspirin use was not associated with greater platelet-to-RBC ratios, which may in part be related to the fact that aspirin does not cause clinically-relevant quantitative platelet defects. Additionally, there were clear differences in transfusion ratios across practice environments. Patients first transfused in the ED received higher ratios of plasma to RBCs, while patients first transfused in the ICU received higher ratios of platelets to RBCs. Future studies are needed to understand transfusion practices and associated clinical outcomes in unique medical environments and by medical professional demographic and training characteristics. This could potentially lead to quality improvement efforts to ensure consistency in blood product utilization across practice locations.
Several prior observational studies focusing primarily on patients with acute intraoperative hemorrhage have demonstrated that higher plasma and platelet to RBC ratios are not associated with improvements in mortality.[7–8,11] The findings of the current investigation limited to non-traumatic, non-intraoperative hemorrhage are consistent with these results. We assessed differences in clinical outcomes to inform hypothesis generation for future clinical trials of optimal transfusion strategies. Outcome differences were limited, particularly with regards to plasma utilization. However, patients receiving platelet to RBC ratios of 0.1-0.9 at 24 hours experienced higher mortality compared to those not receiving platelets, with a similar but non-significant relationship observed in those with ratios >2. Additionally, increasing platelet to RBC ratios were associated with fewer hospital free days. Previous investigations in surgical patients and the critically ill have noted inferior outcomes with platelet transfusion.[18–20] While these findings may represent negative consequences of platelet transfusion, observed associations may alternatively be indicative of greater severity of illness in those receiving platelets with inferior clinical outcomes occurring independently of transfusion. The presented analyses represent associations not causal relationships, and future trials are critically needed to definitively evaluate clinical outcomes across transfusion strategies.
Limitations of this investigation are primarily related to clinical outcome analyses, which must be considered hypothesis-generating. First, the potential for residual confounding exists despite pre-defined statistical adjustment. Second, we were unable to assess several important clinical factors that may influence clinical outcomes such as the severity and rapidity of acute blood loss and the timing of hemorrhage detection. As such, the presented analyses of clinical outcome relationships represent important yet imperfect associations that should not be interpreted causally. Third, the included study cohort was heterogeneous, including both medical and post-surgical patients to reflect real-world clinical practice. The etiologies of hemorrhage and optimal treatment approaches may be distinct in these groups. Fourth, the assessment of multiple clinical outcomes increases type I error rate. Results should be interpreted cautiously with future confirmation. Finally, these results are representative of a large academic medical center. Generalizability to other practice environments is unclear.
In conclusion, transfusion strategies in a diverse cohort of patients with acute hemorrhage occurring outside of trauma and surgery were primarily associated with pre-hemorrhage laboratory values and the hospital environment in which treatment was initiated rather than baseline patient features or anatomic sources of bleeding. Clinical outcomes were not superior in those receiving higher ratios of plasma and platelets to RBCs. Additional investigations are necessary to evaluate the principle drivers of differences in resuscitation strategies across hospital environments and define optimal resuscitation strategies in accordance with unique patient characteristics.
Supplementary Material
Acknowledgments:
Luke Matzek, Ognjen Gajic, Daryl Kor, Matthew Warner: These authors helped in concept and design, data acquisition, interpretation of data, critical writing, revision of intellectual content, and final approval of the manuscript.
Emil Kurian, Ryan Frank, Timothy Weister: These authors helped in analyzing and interpretation of data, critical writing, revision of intellectual content, and final approval of the manuscript.
Financial Support & Declaration of Interests:
This study was made possible by funding from the Mayo Clinic Department of Anesthesiology and Perioperative Medicine and the Critical Care Integrated Multidisciplinary Practice, Rochester, Minnesota. Additionally, research reported in this publication was supported by National Institutes of Health (NIH) R01 grant (HL121232) to Dr. Kor, National Center for Advancing Translational Science (NCATS) KL2 TR002379 to Dr. Warner, and National Heart, Lung, And Blood Institute (NHLBI) of the NIH award number K23HL153310 to Dr. Warner. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
References
- 1.Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. Jama. 2015;313(5):471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borgman MA, Spinella PC, Perkins JG, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63(4):805–813. [DOI] [PubMed] [Google Scholar]
- 3.Holcomb JB, Wade CE, Michalek JE, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248(3):447–458. [DOI] [PubMed] [Google Scholar]
- 4.Holcomb JB, del Junco DJ, Fox EE, et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg. 2013;148(2):127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beckwith H, Manson L, McFarlane C, Reed MJ. A review of blood product usage in a large emergency department over a one-year period. Emerg Med J. 2010;27(6):439–442. [DOI] [PubMed] [Google Scholar]
- 6.Sinha R, Roxby D, Bersten A. Experience with a massive transfusion protocol in the management of massive haemorrhage. Transfus Med. 2013;23(2):108–113. [DOI] [PubMed] [Google Scholar]
- 7.Etchill EW, Myers SP, McDaniel LM, et al. Should All Massively Transfused Patients Be Treated Equally? An Analysis of Massive Transfusion Ratios in the Nontrauma Setting. Crit Care Med. 2017;45(8):1311–1316. [DOI] [PubMed] [Google Scholar]
- 8.Mesar T, Larentzakis A, Dzik W, et al. Association Between Ratio of Fresh Frozen Plasma to Red Blood Cells During Massive Transfusion and Survival Among Patients Without Traumatic Injury. JAMA Surg. 2017;152(6):574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDaniel LM, Neal MD, Sperry JL, et al. Use of a massive transfusion protocol in nontrauma patients: activate away. J Am Coll Surg. 2013;216(6):1103–1109. [DOI] [PubMed] [Google Scholar]
- 10.Morse BC, Dente CJ, Hodgman EI, et al. Outcomes after massive transfusion in nontrauma patients in the era of damage control resuscitation. Am Surg. 2012;78(6):679–684. [PubMed] [Google Scholar]
- 11.Warner M, Frank R, Weister T, et al. Ratios of Plasma and Platelets to Red Blood Cells in Surgical Patients With Acute Intraoperative Hemorrhage. Anesthesia and Analgesia. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. [DOI] [PubMed] [Google Scholar]
- 13.Savage SA, Zarzaur BL, Croce MA, Fabian TC. Redefining massive transfusion when every second counts. J Trauma Acute Care Surg. 2013;74(2):396–400; discussion 400-392. [DOI] [PubMed] [Google Scholar]
- 14.Meyer DE, Cotton BA, Fox EE, et al. A comparison of resuscitation intensity and critical administration threshold in predicting early mortality among bleeding patients: A multicenter validation in 680 major transfusion patients. J Trauma Acute Care Surg. 2018;85(4):691–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Savage SA, Sumislawski JJ, Zarzaur BL, et al. The new metric to define large-volume hemorrhage: results of a prospective study of the critical administration threshold. J Trauma Acute Care Surg. 2015;78(2):224–229; discussion 229-230. [DOI] [PubMed] [Google Scholar]
- 16.Chute CG, Beck SA, Fisk TB, Mohr DN. The Enterprise Data Trust at Mayo Clinic: a semantically integrated warehouse of biomedical data. J Am Med Inform Assoc. 2010;17(2):131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herasevich V, Kor DJ, Li M, Pickering BW. ICU data mart: a non-iT approach. A team of clinicians, researchers and informatics personnel at the Mayo Clinic have taken a homegrown approach to building an ICU data mart. Healthc Inform. 2011;28(11):42, 44–45. [PubMed] [Google Scholar]
- 18.Warner M, Jia Q, Clifford L, et al. Preoperative platelet transfusions and perioperative red blood cell requirements in patients with thrombocytopenia undergoing noncardiac surgery. Transfusion. 2016;56:682–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weil I, Kumar P, Seicean S, Neuhauser D, Seicean A. Platelet count abnormalities and perioperative outcomes in adults undergoing elective, non-cardiac surgery. PLoS One. 2019;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glance L, Blumberdg N, Eaton M, et al. Preoperative thrombocytopenia and postoperative outcomes after noncardiac surgery. Anesthesiol. 2014;120:62–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


