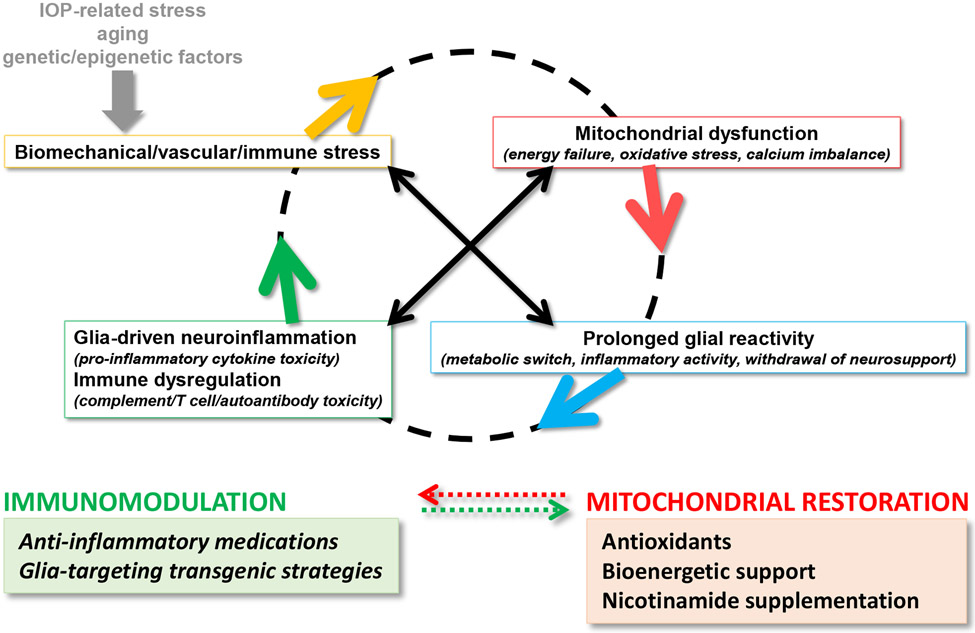
Figure 1. Converging etiological paths of glaucomatous neurodegeneration.
Mitochondrial dysfunction and glia-driven neuroinflammation present interdependent pathogenic processes. Major stressors in glaucoma, including intraocular pressure (IOP) elevation and aging, along with genetic/epigenetic susceptibilities, create biomechanical, vascular, and/or immune stress on RGCs. While dysfunctional mitochondria induce glial inflammatory responses, pro-inflammatory mediators further impair mitochondria, thereby feeding into a vicious cycle that amplifies neurodegeneration in glaucoma. As reviewed herein, preclinical studies explore new strategies for immunomodulation in glaucoma (such as those shown in the green box), which are also expected to protect mitochondria against inflammatory injury. By similarly counting on the interdependence between mitochondrial dysfunction and neuroinflammation, inflammatory outcomes can also be modulated by the treatments targeting mitochondrial dysfunction (such as those shown in the red box, which are tested in clinical studies).