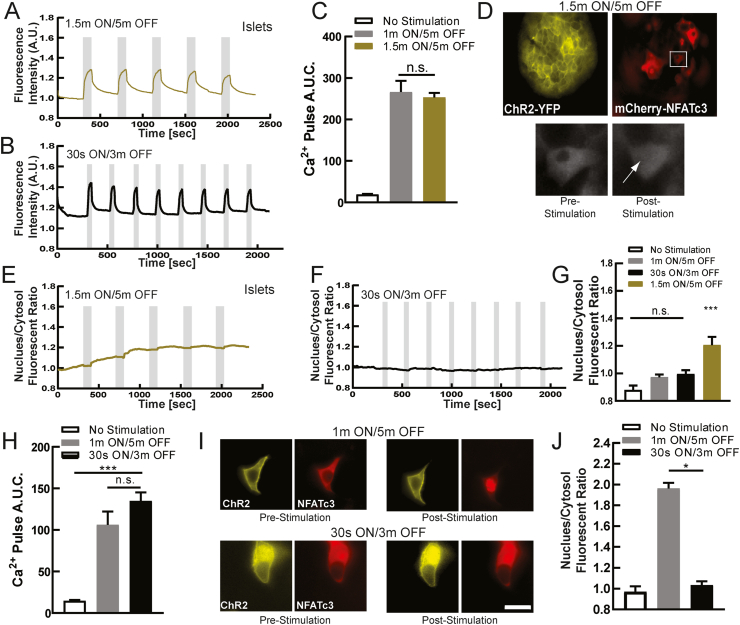
Figure 2.
Dynamic Ca2+changes via optogenetics activates NFATc3. A) Mean Ca2+ changes in mouse islets expressing ChR2(H134R) in the beta-cell, at 2 mM glucose following 1.5 min ON/5 min OFF optical stimulation protocol. Grey indicates duration of optical stimulation. B) As in A for 30 s ON/3 min OFF optical stimulation protocol. C) Area under the curve (AUC) for each stimulation pulse protocol, as well as time course lacking stimulation. D) Representative images of ChR2-expressing islet infected with mCherry-NFATc3, demonstrating NFATc3 translocation following optical stimulation (1.5 min ON/5 min OFF). E) Mean time-course of mCherry-NFATc3 nucleus/cytosol fluorescent ratio following the 1.5 min ON/5 min OFF optical stimulation pulse protocol. F) As in E for the 30 s ON/3m OFF protocol. G) Mean nuclear-cytoplasmic mCherry-NFATc3 ratio 30 min after optical stimulation. H) Area under the curve (AUC) of Ca2+ changes for stimulation pulse protocols (1 min ON/5 min OFF or the 30 s ON/3 min OFF), as well as time-course lacking stimulation, in MIN6 cells transfected with ChR2. I) Representative images of ChR2-expresisng MIN6 cell transfected with mCherry-NFATc3, demonstrating NFATc3 translocation following optical stimulation. J) Mean nuclear-cytoplasmic mCherry-NFATc3 ratio 30 min after optical stimulation in MIB6 cells. Statistical analysis was done using ANOVA with Tukey HSD post hoc test. ∗, ∗∗, and ∗∗∗ represent p = 0.05, p = 0.005, p = 0.0005, respectively. Data in C averaged over n = 4 mice (n = 6 for 1.5 min/5 min; 15,28 islets); data in G averaged over n = 4 mice (n = 6 for 1m/5m; 13,15 islets); data in H averaged over n = 10,12 plates (29,27 cells); data in J averaged over n = 10,24 plates (30,55 cells).