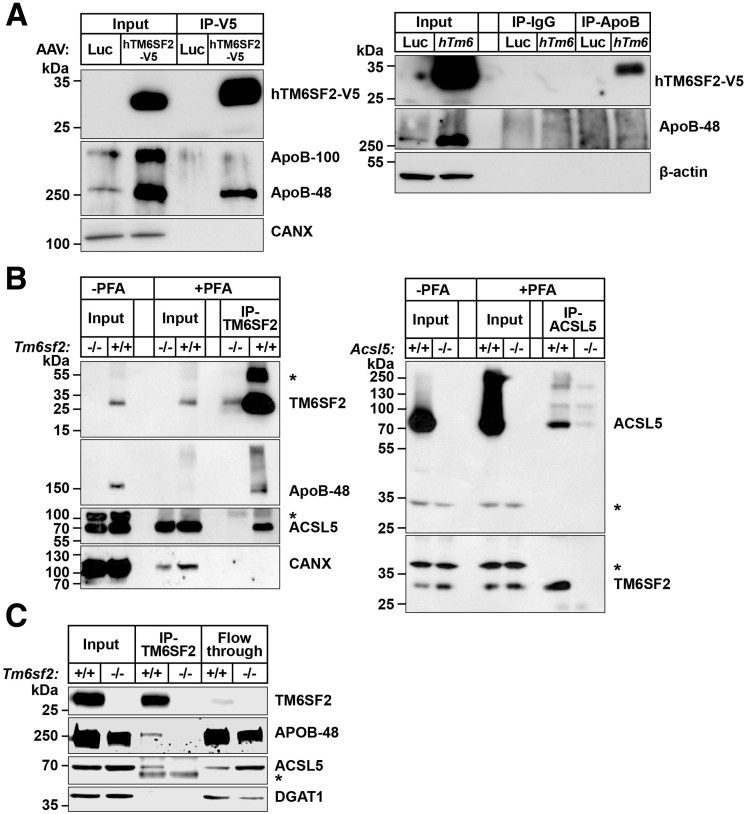
Figure 11.
Co-immunoprecipitation of TM6SF2 with ApoB and ACSL5. (A) Hepatic lysates from mice expressing adeno-associated virus (AAV)–human V5-tagged TM6SF2 (hTM6SF2-V5) or AAV-luciferase were precleaned with A/G agarose beads and then (500 μg, input) was incubated with anti-V5 agarose beads (right) or with rabbit anti-mouse ApoB polyclonal antibody (Abcam Cambridge MA) at 4°C overnight. Input (10%) and IP samples were size-fractionated using SDS–polyacrylamide gel electrophoresis (4%–12%) and immunoblotting was performed as described in the Methods section. (B) Mouse intestinal epithelial cells were incubated ± PFA (1%) and then quenched with 1.25 mol/L glycine. Homogenates from PFA-treated cells (PFA-Input) were precleaned with A/G agarose beads and then 500 μg was incubated with mouse anti-mouse TM6SF2 mAb (8B3) (left) or rabbit anti-mouse ACSL5 (25D12) (right). (C) Co-immunoprecipitation of TM6SF2 with ApoB and ACSL5 from rat intestinal epithelial cells. Samples were handled as described in panel A except cells were homogenized in lysis buffer (1% digitonin, 5 mmol/L EDTA, 5 mmol/L ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid plus PI) and a total of 500 μg of the lysate (input) was incubated with rabbit anti-rat TM6SF2 antibody (505E (25 μg) at 4°C overnight. The mixture then was incubated with Dynabead protein G at 4°C for 2 hours. Samples were collected as described in the Methods section. Fraction (10%) were subjected to immunoblot analysis. ∗Nonspecific band. All experiments were repeated at least once, and the results were similar. CANX, calnexin.