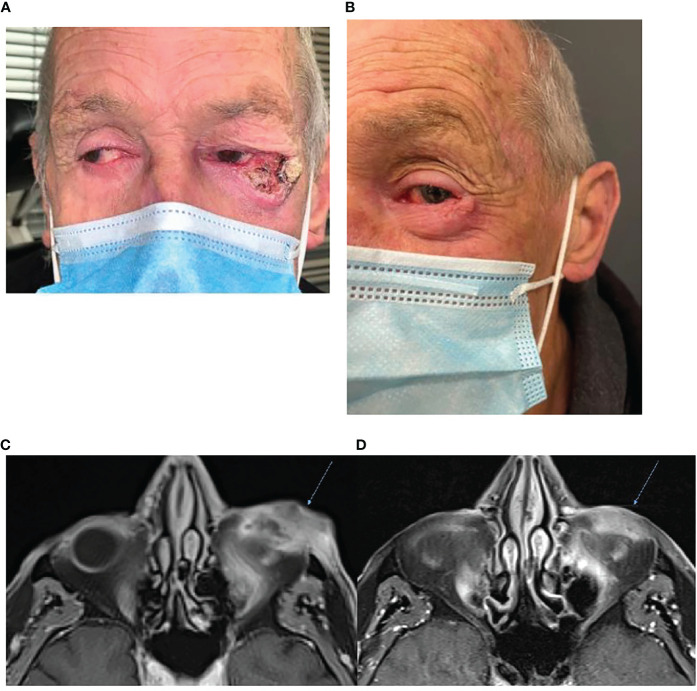
Figure 1.
(A) Baseline photography of left lateral canthus CSCC prior to commencement of immunotherapy. (B) Photography of the lesion post two cycles of cemiplimab 350 mg/3-weekly demonstrating an excellent clinical response to therapy. (C) Baseline, left, MRI post-contrast T1 Fat Sat demonstrating 38 × 18-mm inferolateral canthus enhancing mass. (D) MRI post contrast T1 Fat Sat, post three cycles of cemiplimab 350 mg/3-weekly demonstrating reduced size and enhancement.