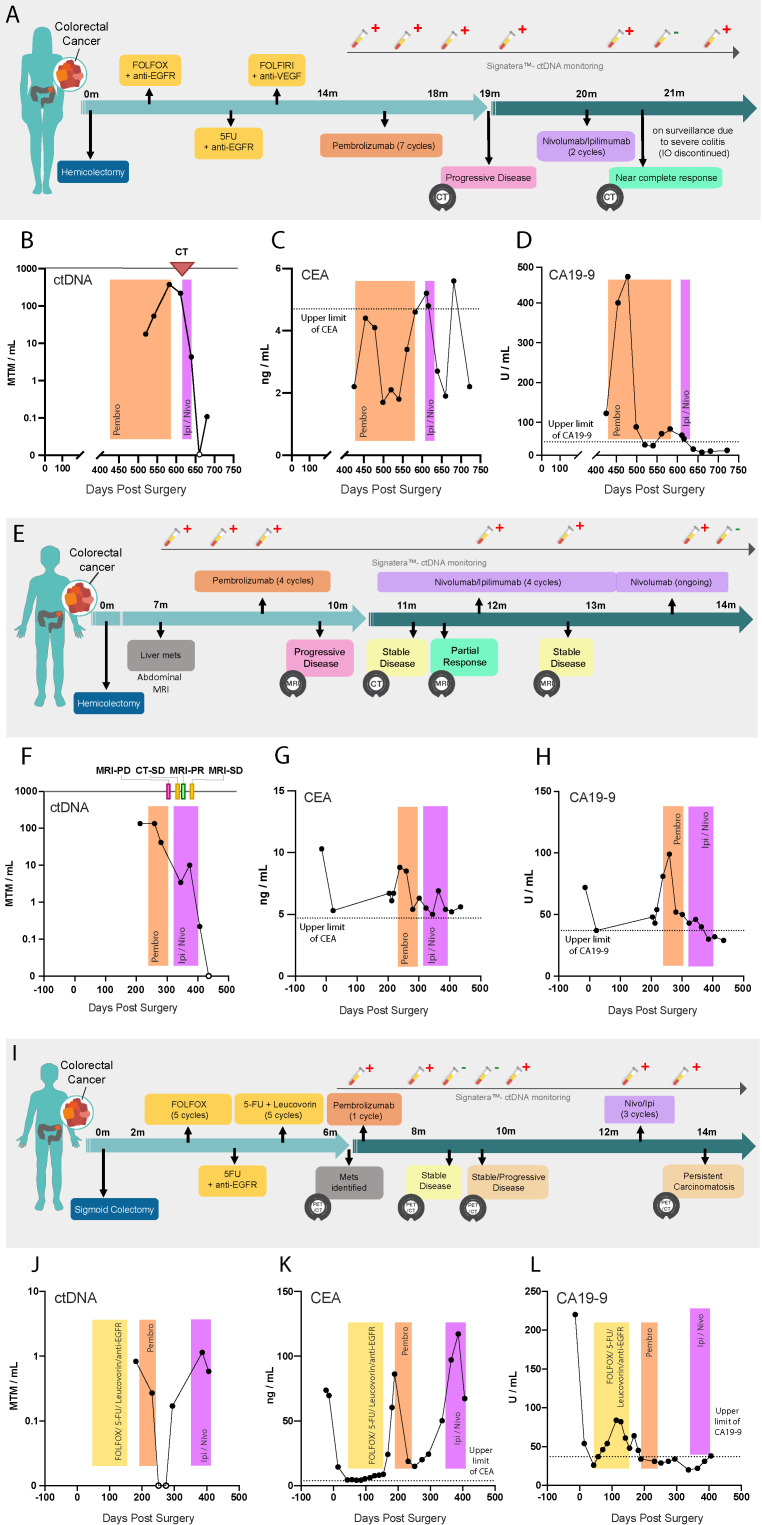
Figure 1.
Clinical course and ctDNA, CEA and CA 19-9 monitoring. (A–D) Case #1, (E–H) Case #2, and (I–L) Case #3 patient clinical course and biomarker levels over time are represented. (A, E, I) Patient plots, providing details on the timeline of treatments administered, PET/CT scanning, and ctDNA monitoring. (B, F, J) ctDNA, (C, G, K) CEA and (D, H, L) CA 19-9 levels over time are represented, measured in number of days since surgical resection of the primary tumor. PET and CT scans, relapse, surgery, and therapeutic treatment windows are represented. CA, carbohydrate antigen; CEA, carcinoembryonic antigen; ctDNA, circulating tumor DNA; MTM, mean tumor molecules; PD, progressive disease; PET/CT, positron emission tomography/computed tomography;; PR, partial response; SD, stable disease 5FU, 5-fluorouracil,