Abstract
Objective
Cartilage repair strategies have seen improvement in recent years, especially with the use of scaffolds that serve as a template for cartilage formation. However, current fixation strategies are inconsistent with regards to retention, may be technically challenging, or may damage adjacent tissues or the implant itself. Therefore, the goal of this study was to evaluate the retention and repair potential of cartilage scaffolds fixed with an easy-to-implement bioresorbable pin.
Design
Electrospun hyaluronic acid scaffolds were implanted into trochlear groove defects in 3 juvenile and 3 adult pigs to evaluate short-term retention (2 weeks; pin fixation vs. press-fit and fibrin fixation) and long-term repair (8 months; scaffold vs. microfracture), respectively.
Results
For the retention study, press-fit and fibrin fixation resulted in short-term scaffold dislodgment (n = 2 each), whereas pin fixation retained all scaffolds that were implanted (n = 6). Pin fixation did not cause any damage to the opposing patellar surface, and only minor changes in the subchondral bone were observed. For long-term repair, no differences were observed between microfracture and scaffold groups, in terms of second-look arthroscopy and indentation testing. On closer visualization with micro computed tomography and histology, a high degree of variability was observed between animals with regard to subchondral bone changes and cartilage repair quality, yet each Scaffold repair displayed similar properties to its matched microfracture control.
Conclusions
In this study, pin fixation did not cause adverse events in either the short- or the long-term relative to controls, indicating that pin fixation successfully retained scaffolds within defects without inhibiting repair.
Keywords: cartilage, fixation, microfracture, animal model, subchondral bone
Introduction
Articular cartilage injuries represent one of the most common intra-articular orthopedic pathologies. In fact, a study determined that greater than 60% of arthroscopies showed articular cartilage damage, 1 many times without symptoms. Unfortunately, these injuries, in addition to those that are debrided (removal of damaged tissue),2,3 often progress in size and severity, continue to erode and deteriorate, and frequently culminate in joint-wide cartilage erosion and the need for total joint replacement. Current techniques to replace this damaged cartilage include microfracture4,5 and autologous chondrocyte implantation (ACI),6,7 utilizing the patient’s own marrow or cartilage cells, respectively, to fill the void. While these approaches can provide some measure of tissue restoration, they do not result in regenerate tissue that matches the native tissue mechanical properties, and so may be susceptible to wear. 8
For this reason, a number of methods and techniques to regenerate functional cartilage tissue are being developed, with many premised on a biomaterial support. One such technique is autologous matrix-induced chondrogenesis (AMIC), 9 which combines marrow recruitment via microfracture with a collagen membrane scaffold. Results from this approach showed considerable improvement over microfracture alone. 10 Likewise, recent modifications to chondrocyte delivery, including matrix-assisted autologous chondrocyte implantation (MACI), involve the preculture of chondrocytes on a simple membrane to localize cell delivery.11,12 In addition to these relatively simple approaches that incorporate clinically used materials and cells, an array of tissue-engineered cartilage implants have been developed utilizing novel materials, bioactive factors, and various cell combinations.13-15 However, for any of these materials/constructs to play a functional role in cartilage repair, fixation into the complex loading environment of the joint will be essential.
A number of fixation techniques have been proposed. Simple fixation techniques (e.g., press-fitting, fibrin glue) may be appropriate for some indications, particularly when the treated defect is of significant depth and surrounded by healthy thick cartilage. When this is not the case, however, there is a high likelihood of scaffold displacement or dislodgement,16,17 likely due to the high shear environment in the joint. 18 On the other hand, rigid fixation techniques (e.g., bone anchors, transosseous or transchondral sutures) may provide superior fixation, but these are more invasive,16,19 with the potential for damage to the subchondral bone, adjacent cartilage, and opposing articular surface. Furthermore, many of these fixation methods can be technically difficult and time-consuming to implement, and some may disrupt cartilage formation by virtue of the volume they occupy in the repair environment. The ideal method for fixation of scaffolds and implants for cartilage regeneration would provide rigid fixation without causing adverse events in the adjacent tissues. The purpose of this study was to evaluate poly-lactide pin fixation of a cartilage repair scaffold (methacrylated hyaluronic acid; MeHA), paying specific attention to the (1) short-term retention of scaffolds and (2) long-term cartilage repair and subchondral bone response in a Yucatan minipig femoral trochlear defect model.
Methods
Study Design
Evaluation of pin fixation ( Fig. 1A ) was performed in 2 separate studies ( Fig. 1B ): a 2-week retention study (“Retention”) in juvenile porcine animals and an 8-month repair study (“Repair”) in adult porcine animals. Juvenile animals were selected for the short-term study to allow bilateral procedures (hence doubling the number of defects) due to their ability to recover, and adult animals were chosen for the long-term study so that a developing joint and lack of tidemark did not confound results. 20 For the Retention study, four small 4-mm-diameter full-thickness chondral defects were created in the femoral trochlea of 3 juvenile animals unilaterally, for a total of 12 defects. All defects received a scaffold, with fixation by press-fit (n = 2), fibrin glue (n = 2), or pin fixation (n = 8). At the termination of the study, defects were analyzed grossly, with micro computed tomography (µCT), and histologically. Subsequently, for the Repair study, two large 6 mm × 12 mm oblong full-thickness defects were created in the trochlea of 3 adult animals, with 1 defect receiving microfracture (MFx) as a control, and the other receiving microfracture with scaffold (Scaffold) fixed by 2 pins. Defects from the Repair study were evaluated with second-look arthroscopy at 4 months (recovery procedure) and 8 months (terminal), analyzed grossly, and subjected to mechanical testing, µCT, and histology.
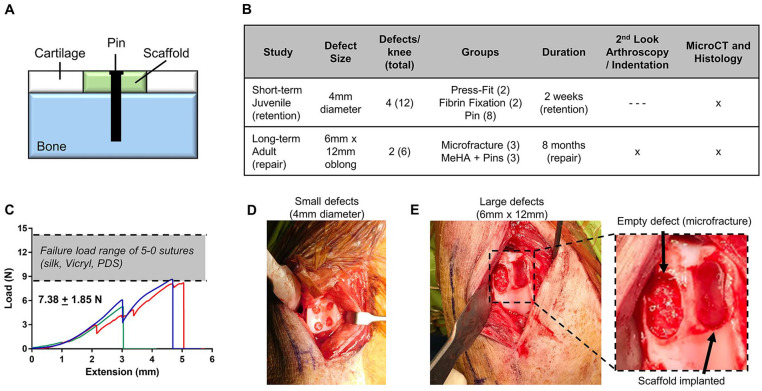
Figure 1.
(A) Schematic of pin-based fixation of scaffolds for full-thickness cartilage defects. (B) Study design outlining the Retention and Repair studies with regards to defect size, number of defects per knee, groups, duration, and analyses. (C) Load versus extension curves of pin pullout mechanics. (D) Four small trochlear defects in juvenile animals for Retention study and (E) 2 large trochlear defects in adult animals for Repair study.
Pin and Scaffold Properties
This study utilized bioresorbable PLDLLA (poly(l-lactide-co-d,l-lactide)) pins (Aesculap FR736, Center Valley, PA) with pin diameter of ~0.90 mm, head diameter of ~1.25 mm, and length of ~6 mm. Resorption of PLDLLA typically occurs on the order of months,21,22 fitting the timeline of the long-term study. For implantation, a pilot hole was first created in the subchondral bone. Next, a scaffold was implanted into the full-thickness defect. A 3-pronged fixation guide (Aesculap FR720, Center Valley PA) was placed on top of the scaffold within the defect, and a pin was placed within the guide, pushed through the scaffold, and tapped into the subchondral bone with a mallet. Biomechanical tests were performed to assess the failure load of the pin in ex vivo porcine osteochondral samples. Pins were inserted into explants with a loop of suture around the head. The suture was tensioned at a rate of 0.05 mm/s with an Instron mechanical testing machine until pin failure or pull-out (n = 3). Fixation failed at an average load of 7.38 N ( Fig. 1C ), with all 3 tests resulting in pin fracture at the head/pin interface. These values approached the range of failure loads for 5-0 sutures, 23 which are stronger than the 6-0 and 7-0 sutures typically used in cartilage repair studies.24,25
The scaffolds in this study were composed of methacrylated hyaluronic acid (MeHA). 26 MeHA was synthesized from hyaluronic acid (~75 kDa, Lifecore Biomedical, Chaska, MN) by methacrylation with methacrylic anhydride to a modification of 42%, as measured by nuclear magnetic resonance (NMR). A solution consisting of MeHA (4% w/v), polyethylene oxide (PEO; 2% w/v), and Irgacure 2959 photoinitiator (0.05% w/v) in DI H2O was loaded into a 10-mL syringe and electrospun to produce nanofibrous mats (500-600 µm thickness), as reported previously.27,28 Mats were purged with nitrogen gas and cross-linked with ultraviolet light exposure (10 mW/cm2) for 15 minutes on each side. For the Retention study, a 4-mm biopsy punch was used to extract scaffolds for implantation. For the Repair study, a 6 mm × 12 mm metal template with rounded edges was placed over the mat, and a surgical blade was used to cut scaffolds to size. All scaffolds were sterilized with ultraviolet light prior to implantation.
Surgical Protocol
All animal studies were performed under an Institutional Animal Care and Use Committee–approved protocol. Three juvenile (~30 kg, 6-8 months old) and 3 adult (~60 kg, 12-14 months old) Yucatan minipigs 29 were subject to unilateral femoral trochlear defects.20,30 Animals were anesthetized and the right stifle joint was shaved, cleaned, and surgically prepared. Through a medial patellar arthrotomy, 31 the patella was dislocated laterally. In the juvenile animals (Retention study), four 4-mm-diameter full-thickness defects were created in the right trochlea via a biopsy punch ( Fig. 1D ). In each of the 12 defects (3 animals × 4 defects), 3 microfracture holes were created with a surgical awl (0.8 mm diameter × ~2 mm deep) followed by placement of 4-mm scaffolds with no additional fixation (press-fit), fixation with fibrin glue (Tisseel, Baxter), or pin fixation. For the Retention study with small defect, 1 pin was used to secure scaffolds, placed at the center of the defect (pin head constituted 9.77% of the defect surface). In the adult animals (Repair), two 6 mm × 12 mm defects were created in the right trochlea, one on each facet. For each defect, a 6-mm biopsy punch was used to make 2 tangent circular defects, and defects were cleaned to create an obround-shaped (rectangle with semicircles on either end) defect ( Fig. 1E ). Both defects per knee received microfracture (7 holes evenly spaced throughout defect), with 1 defect per knee serving as a microfracture control (MFx), and the other receiving an obround MeHA scaffold (Scaffold). Scaffolds in the Repair study were fixed with 2 pins, placed roughly at the center of each original 6-mm circle created from biopsy (pin heads constituted 3.82% of the defect surface). Following implantation, the patella was relocated, and the joint capsule, fascia, and skin were closed via suture. Animals received postoperative analgesia, antibiotics, and anti-inflammatories, and resumed unrestricted cage activity within 2 to 3 hours on recovery from anesthesia.
Second-Look Arthroscopy
For the Repair study, at 4 months postoperatively, animals were anesthetized, and right stifle joints were surgically prepared. Medial and lateral subpatellar arthroscopic portals were established with 1-cm incisions. Through these portals, the trochlear defects were visualized and were probed to subjectively evaluate integration with the surrounding tissue, smoothness, and relative stiffness. Arthroscopies were also performed in the same manner on joints at 8 months, posteuthanasia. At both time points, defects were graded with the arthroscopic scoring system detailed by the International Cartilage Repair Society (ICRS Arthroscopic Scoring System; 0-12, worst-best) 32 by 3 independent observers.
Gross Observations and Indentation Testing
Animals in the Retention study were euthanized at 2 weeks postoperatively. Stifle joints were retrieved, and dissected to expose the patellofemoral joint. The trochlea and patella were evaluated for articular damage related to the pin. The defects were imaged macroscopically, and evaluated for scaffold retention. Animals in the Repair study were euthanized at 8 months postoperatively. Following second-look arthroscopy, joints were dissected and defects were photographed. These macroscopic images were divided into 3 regions per defect: proximal, central, and distal. Each region was graded by 3 independent observers with the Goebel scoring system. 33 Repair samples were then embedded in polymethylmethacrylate (PMMA) with the cartilage surface facing upward. The proximal, central, and distal regions were mechanically tested with a rigid 2-mm-diameter spherical indenter. 34 Samples were subjected to 3 steps of 10% strain, with a strain rate of 0.1%/s, and allowed to relax 600 seconds per step. 35 The equilibrium modulus was calculated from the 30% strain step. Samples from both the Retention and Repair studies, with underlying subchondral bone, were then fixed in Carson’s buffered formalin for 48 hours for µCT and histological analyses.
Micro Computed Tomography
Fixed samples were loaded into a chamber with phosphate buffered saline–soaked gauze to keep samples hydrated during scanning. Samples were scanned (SCANCO µCT50, Wayne, PA) at an isotropic voxel size of 6 µm, with the following scan parameters (tube voltage: 45 kVp; current: 133 µA; exposure time: 900 ms × 5 exposure/projection; 3,000 projections). In both the Retention and Repair studies, with anticipated subchondral bone resorption following either microfracture or pin fixation, the volume of bone resorption was estimated. Libre software package ITK-SNAP 36 . was used for the segmentation and calculation of the bone resorption volume (Supplemental Fig. S1). Boundary regions were determined via the classification segmentation mode and bubbles evenly dispersed throughout the resorption region. Bubbles were allowed to expand through an iterative evolution (region competition force = 1.0, smoothing force = 0.5), until they contacted the bony region surrounding the resorption void. Last, the 3D segmentation rendering was trimmed at the cartilage-bone interface to be level with surrounding subchondral bone.
Bone volume fraction (BV/TV) and mean trabecular thickness of subchondral bone surrounding the defect region was also analyzed via the BoneJ 37 plugin in ImageJ, for both the Retention and Repair studies. DICOM (digital imaging and communications in medicine) files of the µCT images were imported as an 8-bit grayscale image sequence into ImageJ. Images were binarized using the default method. For the long-term samples, a 5 mm × 10 mm selection area was centered around the defect midpoint. For the short-term samples, a 4 mm × 7 mm selection area was centered around the defect midpoint. In both cases, analysis began at the defect start point and ended at the defect end point. Bone volume fraction was calculated by taking the resorption volume into account (Equation 1).
| (1) |
Histological Observations
Following µCT analysis, samples from both Retention and Repair studies were transferred to formic acid solution for decalcification for 4 weeks, with solution replaced weekly. Samples were then transferred to 70% ethanol and processed into paraffin (ethanol dehydration, xylene dehydration, paraffin infusion). Paraffin blocks were sectioned to 8µm with samples taken from the middle of defects for the Retention study (circular defects), and proximal-distal sections to visualize the entire 12-mm defect for the Repair study (obround defects). Sections were stained with safranin O/fast green (SO/FG) for matrix and proteoglycan visualization and hematoxylin and eosin (H&E) to assess cell morphology, inflammatory cells, and vascular invasion. Stained sections were imaged on an automated slide scanner (Aperio ScanScope CS2). For the Retention study, sections were analyzed grossly for scaffold retention. For the Repair study, sections were graded in the proximal, central, and distal regions using the ICRS II histological scoring system 38 by 3 independent observers.
Statistical Analysis
Statistics were not performed on samples from the Retention study, as 2 of the groups had small sample sizes (n = 2), and since this study was designed to verify that scaffolds were retained without a significant tissue response. For the Repair study, statistics consisted of a paired t test between MFx and Scaffold outcomes from the same knee. As high animal-to-animal variability was observed, data from all 3 animals in the Repair study are presented. Results were considered significant with P < 0.05. All statistical analyses were performed in GraphPad Prism 6.
Results
Short-Term Retention Study
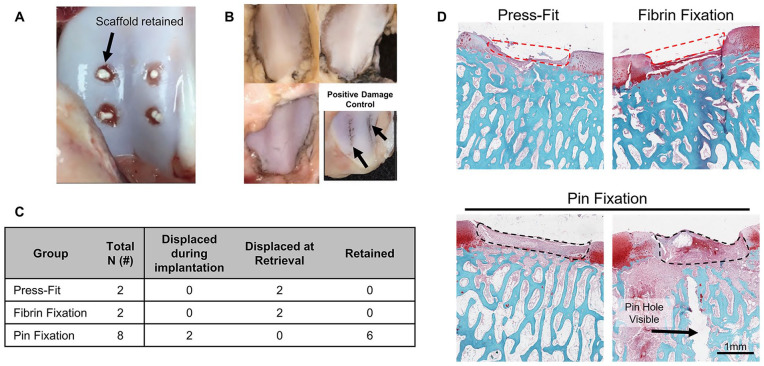
All animals experienced normal recovery and return to weight bearing following surgery. Pin fixation of 2 scaffolds resulted in scaffold rupture and dislodgement during implantation, presumably due to the soft nature of electrospun MeHA scaffolds. Of the remaining 6 scaffolds, all 6 were retained at the defect site at 2 weeks postoperatively ( Fig. 2A ). The opposing articulating patella in all 3 animals showed no observable surface roughening, both before and after India ink staining ( Fig. 2B ). An example of damage due to a surgical curette resulting in positive India ink staining is shown in Fig. 2B (bottom right). All scaffolds in the Press-Fit and Fibrin Fixation groups were displaced at retrieval, indicating lack of fixation with these methods. A summary of retention outcomes is shown in Fig. 2C . These findings were verified histologically ( Fig. 2D ), where a clear full-thickness defect lacking scaffold was observed in Press-Fit and Fibrin Fixation defects, whereas an early repair response was observed within MeHA scaffolds in the Pin Fixation defects.
Figure 2.
Short-term Retention study outcomes. (A) Macroscopic trochlear images showing methacrylated hyaluronic acid (MeHA) scaffolds retained within defects 2-week postoperatively. (B) Macroscopic patellar images showing that pin fixation did not cause damage to the opposing articular surface (shown via lack of India ink staining). The bottom right image represents a patella that was manually damaged with a surgical curette and stained with India ink, as a positive control (black arrows). (C) Table summarizing scaffold retention in the 3 groups. (D) Histological outcomes of Press-Fit, Fibrin Fixation, and Pin Fixation groups. Red dashed line shows region where scaffold was placed but not retained, black dashed line shows retained scaffold, black arrow indicates pin hole. Scale bar represents 1 mm.
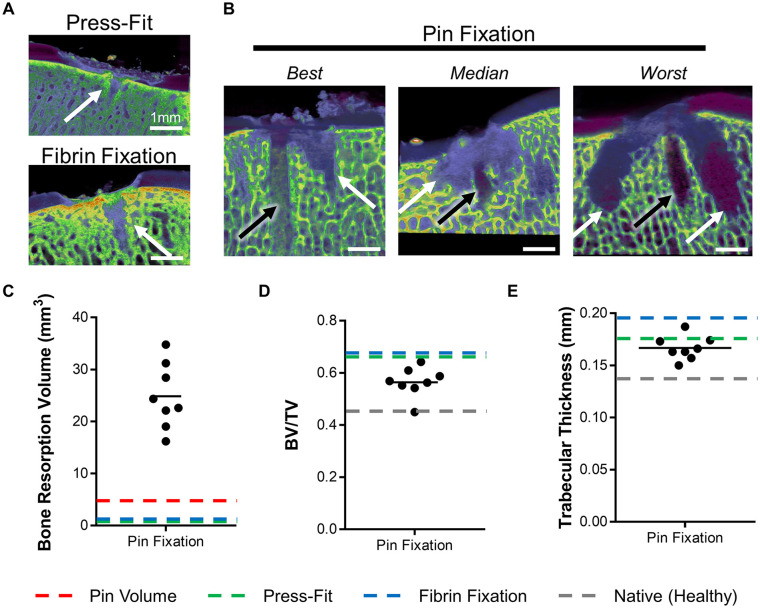
Defects from all 3 groups were also analyzed via µCT to observe early subchondral bone changes. In the Press-Fit and Fibrin Fixation groups, small microfracture “holes” were observed in the subchondral bone ( Fig. 3A —white arrows). In the Pin Fixation group, these microfracture holes, as well as pin holes ( Fig. 3B —black arrows), were observed. Quantification of the volume of bone resorption showed an average resorption of ~25 mm3, beyond the anticipated void left by the pin ( Fig. 3C —red dashed line). Surrounding this void space, bone volume fraction ( Fig. 3D ) and trabecular thickness ( Fig. 3E ) were relatively unaffected by pin fixation.
Figure 3.
Subchondral bone response via micro computed tomography (µCT). (A) Representative images of Press-Fit and Fibrin Fixation defects, and (B) best, median, and worst images of Pin Fixation defects. White arrows indicate microfracture holes, black arrows indicate pin holes. Scale bar represents 1 mm. (C) Bone resorption volume (mm3), (D) bone volume fraction (bone volume/total volume), and (E) trabecular thickness (mm) Pin Fixation groups. Dashed lines represent the mean values for the volume of pin itself (red), or for all mean values for Press-Fit fixation (green), Fibrin fixation (blue), and Native tissue (gray) groups.
Long-Term Repair Study
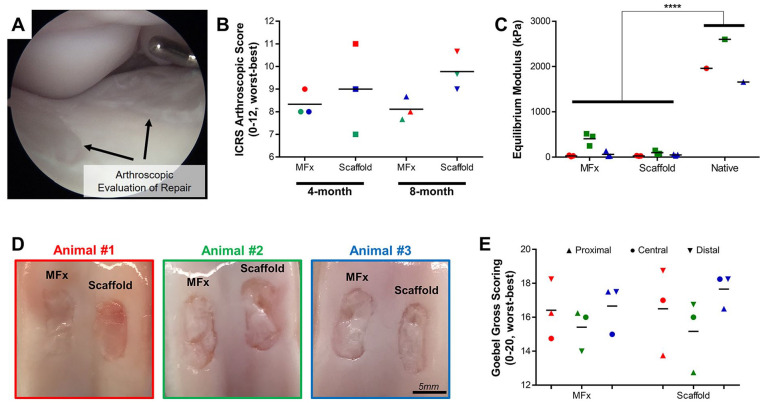
For the long-term repair study, all three adult animals experienced normal recovery and return to weight bearing following both the initial implantation surgery and the second-look arthroscopy performed subterminally (at 4 months). Second-look arthroscopy provided excellent visualization of both defects in the knee ( Fig. 4A ), and verified that all 3 scaffolds were retained. Grading of these defects with the ICRS Arthroscopy Score ( Fig. 4B ) showed no observable differences at 4 months between MFx and Scaffold groups. However, at 8 months, scoring was greater in the Scaffold group compared with MFx in all 3 animals (P = 0.25). Indentation testing showed no differences between MFx and Scaffold groups (P = 0.1174), with both groups being considerably lower (P < 0.0001) than Native tissue ( Fig. 4C ). Macroscopic observation of defects on retrieval showed no differences between groups, both visually ( Fig. 4D ) and with the Goebel gross scoring system ( Fig. 4E ). Interestingly, results varied more by animal than by treatment group, and thus, the data from all 3 animals is presented.
Figure 4.
Long-term macroscopic observations. (A) Sample arthroscopic image showing visualization of defects. (B) ICRS Arthroscopy Score (0-12, worst-best) at 4 and 8 months, depicted by animal (#1 = red, #2 = green, #3 = blue). (C) Indentation testing of microfracture (MFx) and Scaffold conditions (n = 3 locations from 3 defects) compared with Native (n = 1 location from 3 healthy samples). (D) Macroscopic visualization of MFx and Scaffold defects. Scale bar = 5 mm. (E) Goebel gross scoring (0-20, worst-best) of proximal (▲), central (•), and distal (▼) regions of all defects.
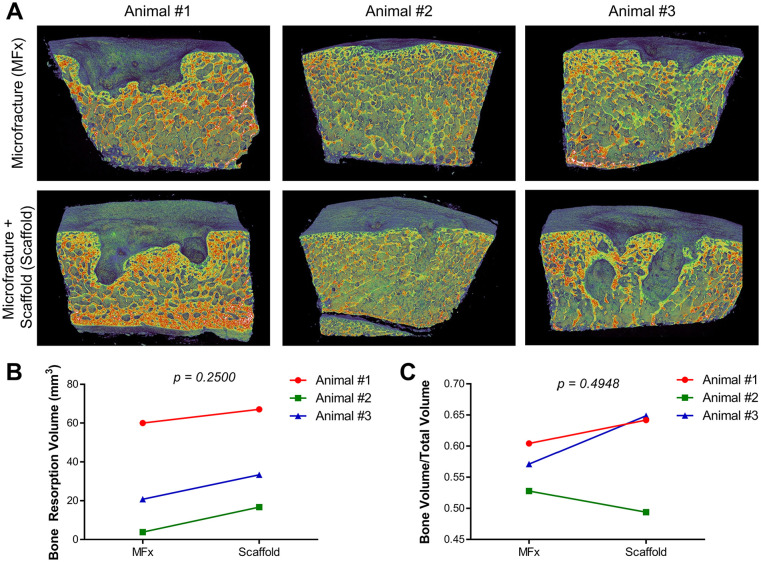
The subchondral bone was evaluated with µCT at 8 months to determine long-term response to the pin fixation and to the treatment approach. Between the three animals, this response was highly variable, but was relatively consistent between defects within the same animal ( Fig. 5A ). Animal 1 showed a significant amount of subchondral bone resorption under the defect in both MFx and Scaffold conditions. Animal 2 displayed little-to-no visual subchondral bone response in the MFx, and only small pinholes were observed in the Scaffold defect for this animal. Animal 3 displayed an intermediate response between Animals 1 and 2. The volume of subchondral bone resorption ( Fig. 5B ) was not significantly different between MFx and Scaffold groups (P = 0.2500) and showed marked variability between animals. The increase in resorption volume varied between 7 and 13 mm3, in the range of the volume of 2 pins. Bone volume fraction ( Fig. 5C ) was not different between MFx and Scaffold groups, indicating that the pins did not cause an adverse long-term response.
Figure 5.
Long-term subchondral bone response. (A) Mid-defect images of subchondral bone from microfracture (MFx) and Scaffold defects in all 3 animals. (B) Bone resorption volume (mm3) and (C) bone volume fraction (bone volume/total volume).
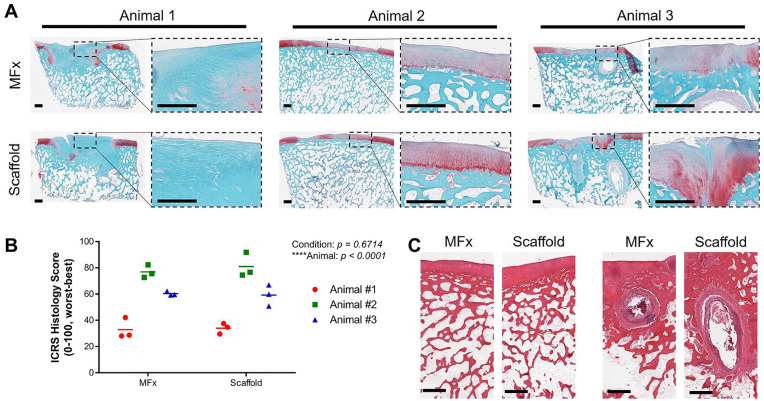
Finally, samples were processed for histological analysis. Similar to µCT findings, repair quality was variable between animals, with Animal 1 showing fibrous tissue deposition within the defect, little to no proteoglycan staining, and a large subchondral void filled with fibrous tissue ( Fig. 6A —left). These findings were consistent between the MFx and Scaffold defects. In contrast, both the MFx and Scaffold treated defects in Animal 2 showed areas of hyaline-like cartilage, and the subchondral bone remained mostly intact. Of note, the Scaffold group showed slightly stronger proteoglycan staining ( Fig. 6A —middle). Finally, Animal 3 showed repair quality that was intermediate between Animals 1 and 2. These results were confirmed with histological scoring ( Fig. 6B ) finding that treatment was not a statistically significant factor (P = 0.6714), but that animal was (P < 0.0001). These results indicate that pin fixation did not negatively influence cartilage repair quality relative to microfracture, and that the repair and subchondral bone quality were more dependent on variations in animal to animal response. A closer examination of H&E-stained sections of Animals 2 and 3 ( Fig. 6C —left and right, respectively), showed similar subchondral bone structure between conditions, but not between animals. In fact, Animal 3 exhibited subchondral bone cyst formation in the distal aspect of the defect in both MFx and Scaffold conditions.
Figure 6.
Long-term histological data. (A) Sections stained for safranin O/fast green for each animal (1, 2, 3) and condition (MFx, Scaffold). Visualization of the whole defect, and a zoom-in of the cartilage are shown. Scale bar = 1 mm. (B) ICRS II histological scoring of the proximal, central, and distal regions of defects, reported for each animal and condition. (C) Hematoxylin and eosin (H&E)-stained sections of defects from Animal 2 (left) and Animal 3 (right), showing similarities between conditions in the same animal. Animal 3 showed subchondral cyst formation in the distal aspect of defects in both MFx- and Scaffold-treated defects.
Discussion
Fixation is a key consideration in the efficacy of repair and regeneration techniques for cartilage replacement. Obviously, fixation must retain scaffolds in the defect site, but at the same time, must not inhibit tissue repair. A variety of fixation techniques have been employed both preclinically and clinically, ranging from simple “liquid” based techniques (e.g., fibrin, platelet-rich plasma) to more complex sutures and anchors. In this study, we tested an easy-to-implement bioresorbable pin for cartilage scaffold fixation. Our findings show successful retention of scaffolds in the short-term, and in the long-term avoided adverse effects on defect repair relative to microfracture controls. Interestingly and not anticipated, both subchondral response and cartilage repair quality were more dependent on the animal rather than the treatment condition, suggesting that the extent of cartilage repair with a given therapy may be more subject driven than therapy driven.
Without fixation with bioresorbable pins, scaffolds were not retained at the defect site. Press-fit fixation may be adequate for certain osteochondral tissue grafts, 39 but its use with softer tissue engineered constructs or repair membranes can result in detachment. 17 This can likely be attributed to the high shear forces within the patellofemoral joint under normal physiologic activity. 40 Moreover, softer scaffolds may undergo larger strains and displacements, and press-fit fixation generally results in pullout loads of less than 1 N. 41 Additional fixation with fibrin glue over the top of defects only marginally improves fixation strength (~2.5 N) and does not improve scaffold retention, both in this study and the literature.19,42 In addition to limited efficacy in enhancing scaffold retention in animal studies, the use of fibrin glue as an adjuvant to scaffold-based bone and cartilage repair may reduce repair tissue quality. The supraphysiologic concentrations of thrombin and fibrinogen that make these products useful as hemostatic agents may prove detrimental to tissue regenerative procedures that rely on host cell migration into a scaffold.43,44 Furthermore, the dense clots formed by fibrin glue may stimulate a prolonged inflammatory response due to resistance to neutrophilic infiltration and degradation during normal wound healing processes.45,46 Other solution-based approaches, such as platelet-rich plasma have also failed in large-animal models, 47 and thus more rigid techniques with greater integrity are required. While the aforementioned fixation techniques do not provide adequate fixation strength, they are relatively easy and quick to implement. On the other end of the spectrum, direct fixation to the surrounding tissues (adjacent cartilage or underlying subchondral bone) can significantly improve mechanics. For example, Knecht et al 41 showed that chondral suture fixation of collagen membranes resulted in an average fixation load of 9.29 N, and Friedman et al 19 showed that bone anchor fixation into the subchondral bone resulted in an average failure load of 6.96 N. However, these approaches can be technically challenging and time-consuming, 16 and cause morbidity and marked changes in the adjacent tissues. Bone anchors can leave large subchondral voids within months following implantation.19,48 In our study, the resorbable bone pins were easily implemented, and generated a high failure load (7.38 N) in line with previous suture and bone anchor fixation studies. This high failure load likely allowed scaffolds to be retained within defects in the early stages of healing, which is when it is most important. 47 Furthermore, while these more invasive fixation techniques had an impact on subchondral bone response over defect or microfracture controls, the use of the bioresorbable pins in this study did not affect repair relative to controls. Prior studies in caprine 49 and equine 50 models fixed scaffolds with a resorbable polydiaxanone-polyglycolic acid (PGA) staple, similar to the pin in this study, and demonstrated its retentive capacity with comparable levels of subchondral bone changes.
In terms of long-term repair quality, the MeHA scaffolds did not significantly improve healing over microfracture controls. These findings are consistent with previous evidence that shows slight, if any, improvement with electrospun MeHA scaffolds. 51 Notably, addition of bioactive factors (such as transforming growth factor beta) can markedly improve repair using these scaffolds.15,51 While the cartilage formed in this study is not ideal, it should be noted that pin fixation did not negatively affect the quality of cartilage repair, relative to the microfracture control within the same animal. In fact, the 3 animals from the long-term Repair study showed markedly different subchondral bone response and cartilage repair quality, irrespective of the condition. Both defects in the first animal displayed significant subchondral bone resorption, with large bone resorption “craters” that were filled in with fibrous, proteoglycan-deficient tissue. The defects of the second animal displayed little to no subchondral bone changes, with only slight resorption surrounding the original pin holes. The repair tissue in this animal was the highest quality, with ICRS histological scores approaching healthy native tissue (score of 100). Both defects in the third animal showed an intermediate level of subchondral bone changes and cartilage repair quality, with values in between those of the first 2 animals. Thus, it appears that the level of subchondral resorption and remodeling may be influential in the state of the regenerate tissue, or vice versa.
While not initially considered a study goal, the variability observed between animals was perhaps the most interesting result. These findings may be used to inform clinical decisions, demonstrating suggest that even microfracture treatment could have considerable unpredictability between patients. 52 Furthermore, we showed that arthroscopic visualization of cartilage repair tissue is not always indicative of the true characteristics (mechanics, µCT, histology) of newly formed cartilage, given that the Scaffold defect with the best arthroscopic score was in fact the worst defect in terms of subchondral bone resorption and histological scoring. Thus, beyond the evaluation of pin fixation, the experimental findings of this study may also provide some clinical insight into variability in repair response.
This study did present a few limitations. First and foremost, the sample size was certainly limited (n = 3 for the Retention and Repair studies, each). However, this study was experimental in nature, as is necessary to evaluate new fixation techniques. Second, only 1 scaffold type was chosen; the electrospun MeHA scaffolds used in this study are relatively soft compared with others used in cartilage tissue engineering.14,35,53 Fixation loads of these more robust scaffolds are certainly higher 41 and thus the retention of these scaffolds is expected to be even better. Third, we tested pin fixation on the trochlea, which may not experience the same levels of stress as the femoral condyle. Thus, future studies may need to verify the capacity for pin fixation in the tibiofemoral joint, as well as evaluate the surface opposing the pin due to the higher susceptibility to kissing lesions. Finally, for the Repair study, another limitation was that fixation without pins was not performed, as a negative control. However, based on the short-term Retention results, a long-term animal study without adequate scaffold retention would have been wasteful.
Conclusions
This study demonstrated the ability of an easy-to-implement resorbable pin to anchor tissue engineering scaffolds, retain them within a full-thickness cartilage defects, and allow long-term repair without adverse effects. Interestingly, long-term cartilage repair and subchondral bone response depended more on animal than on treatment type (microfracture vs. scaffold). These results provide supportive data regarding the use of bioresorbable pins for cartilage repair scaffold fixation without adverse events, and may motivate the need to consider inherent individual subject variability in cartilage treatment strategies.
Supplemental Material
Supplemental material, Supplemental for Resorbable Pins to Enhance Scaffold Retention in a Porcine Chondral Defect Model by Jay M. Patel, Mackenzie L. Sennett, Anthony R. Martin, Kamiel S. Saleh, Michael R. Eby, Blair S. Ashley, Liane M. Miller, George R. Dodge, Jason A. Burdick, James L. Carey and Robert L. Mauck in CARTILAGE
Footnotes
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors would like to thank Robert Spiro and Aesculap for their assistance in acquiring and using the pins in this study. We would also like to thank Matthew Davidson and Claudia Loebel for their help with scaffold fabrication, and University Laboratory Animal Resources (ULAR) at Penn for their assistance with the animal studies. This work was supported by the American Orthopaedic Society for Sports Medicine (AOSSM), the National Institutes of Health (R01 AR077362, R01 AR056145), the Department of Veterans Affairs (IK1 RX003208, IK6 RX003416, I01 RX003375), and the Penn Center for Musculoskeletal Disorders Biomechanics Core (P30 AR069619).
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The are no competing interests that influenced this work. JMP is a consultant for NovoPedics Inc. JLC is a consultant for Vericel and assistant editor of the American Journal of Sports Medicine. RLM is a coeditor for Journal of Orthopaedic Research–Spine.
Ethical Approval: All animal studies were performed under an Institutional Animal Care and Use Committee–approved protocol.
ORCID iD: Jay M. Patel  https://orcid.org/0000-0003-4889-0818
https://orcid.org/0000-0003-4889-0818
Supplementary material for this article is available on the Cartilage website at https://journals.sagepub.com/home/car.
References
- 1. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31 516 knee arthroscopies. Arthroscopy. 1997;13(4):456-60. [DOI] [PubMed] [Google Scholar]
- 2. Laupattarakasem W, Laopaiboon M, Laupattarakasem P, Sumananont C. Arthroscopic debridement for knee osteoarthritis. Cochrane Database Syst Rev. 2008;(1):CD005118. doi: 10.1002/14651858.CD005118.pub2 [DOI] [PubMed] [Google Scholar]
- 3. Spahn G, Hofmann GO, Klinger HM. The effects of arthroscopic joint debridement in the knee osteoarthritis: results of a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2013;21(7):1553-61. doi: 10.1007/s00167-012-2169-1 [DOI] [PubMed] [Google Scholar]
- 4. Gracitelli GC, Moraes VY, Franciozi CES, Luzo MV, Belloti JC. Surgical interventions (microfracture, drilling, mosaicplasty, and allograft transplantation) for treating isolated cartilage defects of the knee in adults. Cochrane Database Syst Rev. 2016;9(9):CD010675. doi: 10.1002/14651858.CD010675.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Solheim E, Hegna J, Inderhaug E, Øyen J, Harlem T, Strand T. Results at 10-14 years after microfracture treatment of articular cartilage defects in the knee. Knee Surg Sports Traumatol Arthrosc. 2016;24(5):1587-93. doi: 10.1007/s00167-014-3443-1 [DOI] [PubMed] [Google Scholar]
- 6. Bentley G, Biant LC, Vijayan S, Macmull S, Skinner JA, Carrington RWJ. Minimum ten-year results of a prospective randomised study of autologous chondrocyte implantation versus mosaicplasty for symptomatic articular cartilage lesions of the knee. Bone Joint J. 2012;94-B(4):504-9. doi: 10.1302/0301-620X.94B4.27495 [DOI] [PubMed] [Google Scholar]
- 7. Peterson L, Vasiliadis HS, Brittberg M, Lindahl A. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med. 2010;38(6):1117-24. doi: 10.1177/0363546509357915 [DOI] [PubMed] [Google Scholar]
- 8. Strauss EJ, Barker JU, Kercher JS, Cole BJ, Mithoefer K. Augmentation strategies following the microfracture technique for repair of focal chondral defects. Cartilage. 2010;1(2_suppl):145-52. doi: 10.1177/1947603510366718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Benthien JP, Behrens P. Autologous matrix-induced chondrogenesis (AMIC): combining microfracturing and a collagen I/III matrix for articular cartilage resurfacing. Cartilage. 2010;1(1):65-8. doi: 10.1177/1947603509360044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Volz M, Schaumburger J, Frick H, Grifka J, Anders S. A randomized controlled trial demonstrating sustained benefit of autologous matrix-induced chondrogenesis over microfracture at five years. Int Orthop. 2017;41(4):797-804. doi: 10.1007/s00264-016-3391-0 [DOI] [PubMed] [Google Scholar]
- 11. Basad E, Ishaque B, Bachmann G, Stü H, Steinmeyer J. Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of cartilage defects of the knee: a 2-year randomised study. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):519-27. doi: 10.1007/s00167-009-1028-1 [DOI] [PubMed] [Google Scholar]
- 12. Brittberg M, Recker D, Ilgenfritz J, Saris DBF; Summit Extension Study Group. Matrix-applied characterized autologous cultured chondrocytes versus microfracture: five-year follow-up of a prospective randomized trial. Am J Sports Med. 2018;46(6):1343-51. doi: 10.1177/0363546518756976 [DOI] [PubMed] [Google Scholar]
- 13. Censi R, Dubbini A, Matricardi P. Bioactive hydrogel scaffolds—advances in cartilage regeneration through controlled drug delivery. Curr Pharm Des. 2015;21(12):1545-55. doi: 10.2174/1381612821666150115150712 [DOI] [PubMed] [Google Scholar]
- 14. Martín AR, Patel JM, Zlotnick HM, Carey JL, Mauck RL. Emerging therapies for cartilage regeneration in currently excluded “red knee” populations. NPJ Regen Med. 2019;4:12. doi: 10.1038/s41536-019-0074-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Patel JM, Saleh KS, Burdick JA, Mauck RL. Bioactive factors for cartilage repair and regeneration: Improving delivery, retention, and activity. Acta Biomater. 2019;93:222-38. doi: 10.1016/j.actbio.2019.01.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Drobnič M, Radosavljevič D, Ravnik D, Pavlovčič V, Hribernik M. Comparison of four techniques for the fixation of a collagen scaffold in the human cadaveric knee. Osteoarthritis Cartilage. 2006;14(4):337-44. doi: 10.1016/j.joca.2005.11.007 [DOI] [PubMed] [Google Scholar]
- 17. Marlovits S, Striessnig G, Kutscha-Lissberg F, Resinger C, Aldrian SM, Vécsei V, et al. Early postoperative adherence of matrix-induced autologous chondrocyte implantation for the treatment of full-thickness cartilage defects of the femoral condyle. Knee Surg Sports Traumatol Arthrosc. 2005;13(6):451-7. doi: 10.1007/s00167-004-0535-3 [DOI] [PubMed] [Google Scholar]
- 18. Wong BL, Bae WC, Gratz KR, Sah RL. Shear deformation kinematics during cartilage articulation: effect of lubrication, degeneration, and stress relaxation. Mol Cell Biomech. 2008;5(3):197-206. [PMC free article] [PubMed] [Google Scholar]
- 19. Friedman JM, Sennett ML, Bonadio MB, Orji KO, Neuwirth AL, Keah N, et al. comparison of fixation techniques of 3D-woven poly(ε-caprolactone) scaffolds for cartilage repair in a weightbearing porcine large animal model. Cartilage. 2018;9(4):428-37. doi: 10.1177/1947603517700953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pfeifer CG, Fisher MB, Saxena V, Kim M, Henning EA, Steinberg DA, et al. Age-dependent subchondral bone remodeling and cartilage repair in a minipig defect model. Tissue Eng Part C Methods. 2017;23(11):745-53. doi: 10.1089/ten.tec.2017.0109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Landes C, Ballon A, Ghanaati S, Ebel D, Ulrich D, Spohn U, et al. Evaluation of the fatigue performance and degradability of resorbable PLDLLA-TMC osteofixations. Open Biomed Eng J. 2013;7:133-46. doi: 10.2174/1874120701307010133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lazennec JY, Madi A, Rousseau MA, Roger B, Saillant G. Evaluation of the 96/4 PLDLLA polymer resorbable lumbar interbody cage in a long term animal model. Eur Spine J. 2006;15(10):1545-53. doi: 10.1007/s00586-006-0145-5 [DOI] [PubMed] [Google Scholar]
- 23. Abiri A, Paydar O, Tao A, LaRocca M, Liu K, Genovese B, et al. Tensile strength and failure load of sutures for robotic surgery. Surg Endosc. 2017;31(8):3258-70. doi: 10.1007/s00464-016-5356-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. DuRaine GD, Arzi B, Lee JK, Lee CA, Responte DJ, Hu JC, et al. Biomechanical evaluation of suture-holding properties of native and tissue-engineered articular cartilage. Biomech Model Mechanobiol. 2015;14(1):73-81. doi: 10.1007/s10237-014-0589-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hunziker EB, Stähli A. Surgical suturing of articular cartilage induces osteoarthritis-like changes. Osteoarthritis Cartilage. 2008;16(9):1067-73. doi: 10.1016/j.joca.2008.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Burdick JA, Chung C, Jia X, Randolph MA, Langer R. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules. 2005;6(1):386-91. doi: 10.1021/bm049508a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim IL, Khetan S, Baker BM, Chen CS, Burdick JA. Fibrous hyaluronic acid hydrogels that direct MSC chondrogenesis through mechanical and adhesive cues. Biomaterials. 2013;34(22):5571-80. doi: 10.1016/j.biomaterials.2013.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sundararaghavan HG, Burdick JA. Gradients with depth in electrospun fibrous scaffolds for directed cell behavior. Biomacromolecules. 2011;12(6):2344–50. doi: 10.1021/bm200415g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cone SG, Warren PB, Fisher MB. Rise of the pigs: utilization of the porcine model to study musculoskeletalbiomechanics and tissue engineering during skeletal growth. Tissue Eng Part C Methods. 2017;23(11):763-80. doi: 10.1089/ten.tec.2017.0227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fisher MB, Belkin NS, Milby AH, Henning EA, Bostrom M, Kim M, et al. Cartilage repair and subchondral bone remodeling in response to focal lesions in a mini-pig model: implications for tissue engineering. Tissue Eng Part A. 2015;21(3-4):850-60. doi: 10.1089/ten.tea.2014.0384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bonadio MB, Friedman JM, Sennett ML, Mauck RL, Dodge GR, Madry H. A retinaculum-sparing surgical approach preserves porcine stifle joint cartilage in an experimental animal model of cartilage repair. J Exp Orthop. 2017;4(1):11. doi: 10.1186/s40634-017-0083-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Smith GD, Taylor J, Almqvist KF, Erggelet C, Knutsen G, Portabella MG, et al. Arthroscopic assessment of cartilage repair: a validation study of 2 scoring systems. Arthroscopy. 2005;21(12):1462-7. doi: 10.1016/j.arthro.2005.09.007 [DOI] [PubMed] [Google Scholar]
- 33. Goebel L, Orth P, Müller A, Zurakowski D, Bücker A, Cucchiarini M, et al. Experimental scoring systems for macroscopic articular cartilage repair correlate with the MOCART score assessed by a high-field MRI at 9.4 T—comparative evaluation of five macroscopic scoring systems in a large animal cartilage defect model. Osteoarthritis Cartilage. 2012;20(9):1046-55. doi: 10.1016/j.joca.2012.05.010 [DOI] [PubMed] [Google Scholar]
- 34. Meloni GR, Fisher MB, Stoeckl BD, Dodge GR, Mauck RL. Biphasic finite element modeling reconciles mechanical properties of tissue-engineered cartilage constructs across testing platforms. Tissue Eng Part A. 2017;23(13-14):663-74. doi: 10.1089/ten.tea.2016.0191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Patel JM, Wise BC, Bonnevie ED, Mauck RL. A systematic review and guide to mechanical testing for articular cartilage tissue engineering. Tissue Eng Part C Methods. 2019;25(10):593-608. doi: 10.1089/ten.TEC.2019.0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, et al. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage. 2006;31(3):1116-28. doi: 10.1016/j.neuroimage.2006.01.015 [DOI] [PubMed] [Google Scholar]
- 37. Doube M, Klosowski MM, Arganda-Carreras I, Cordelières FP, Dougherty RP, Jackson JS, et al. BoneJ: free and extensible bone image analysis in ImageJ. Bone. 2010;47(6):1076-9. doi: 10.1016/j.bone.2010.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mainil-Varlet P, Rieser F, Grogan S, Mueller W, Saager C, Jakob RP. Articular cartilage repair using a tissue-engineered cartilage-like implant: an animal study. Osteoarthritis Cartilage. 2001;9(Suppl A):S6-S15. doi: 10.1053/joca.2001.0438 [DOI] [PubMed] [Google Scholar]
- 39. Duchow J, Hess T, Kohn D. Primary stability of press-fit-implanted osteochondral grafts: influence of graft size, repeated insertion, and harvesting technique. Am J Sports Med. 2000;28(1):24-7. doi: 10.1177/03635465000280011601 [DOI] [PubMed] [Google Scholar]
- 40. Besier TF, Gold GE, Beaupré GS, Delp SL. A modeling framework to estimate patellofemoral joint cartilage stress in vivo. Med Sci Sports Exerc. 2005;37(11):1924-30. doi: 10.1249/01.mss.0000176686.18683.64 [DOI] [PubMed] [Google Scholar]
- 41. Knecht S, Erggelet C, Endres M, Sittinger M, Kaps C, Stüssi E. Mechanical testing of fixation techniques for scaffold-based tissue-engineered grafts. J Biomed Mater Res B Appl Biomater. 2007;83:50-7. doi: 10.1002/jbm.b.30765 [DOI] [PubMed] [Google Scholar]
- 42. Efe T, Füglein A, Heyse TJ, Stein T, Timmesfeld N, Fuchs-Winkelmann S, et al. Fibrin glue does not improve the fixation of press-fitted cell-free collagen gel plugs in an ex vivo cartilage repair model. Knee Surg Sports Traumatol Arthrosc. 2012;20(2_suppl):201-5. doi: 10.1007/s00167-011-1571-4 [DOI] [PubMed] [Google Scholar]
- 43. Brittberg M, Sjögren-Jansson E, Lindahl A, Peterson L. Influence of fibrin sealant (Tisseel®) on osteochondral defect repair in the rabbit knee. Biomaterials. 1997;18(3):235-42. doi: 10.1016/S0142-9612(96)00117-2 [DOI] [PubMed] [Google Scholar]
- 44. Karp JM, Sarraf F, Shoichet MS, Davies JE. Fibrin-filled scaffolds for bone-tissue engineering: an in vivo study. J Biomed Mater Res A. 2004;71(1):162-71. doi: 10.1002/jbm.a.30147 [DOI] [PubMed] [Google Scholar]
- 45. Hanson AJ, Quinn MT. Effect of fibrin sealant composition on human neutrophil chemotaxis. J Biomed Mater Res. 2002;61(3)474-81. doi: 10.1002/jbm.10196 [DOI] [PubMed] [Google Scholar]
- 46. Mancini IAD, Bolaños RAV, Brommer H, Castilho M, Ribeiro A, van Loon JPAM, et al. Fixation of hydrogel constructs for cartilage repair in the equine model: a challenging issue. Tissue Eng Part C Methods. 2017;23(11):804-14. doi: 10.1089/ten.tec.2017.0200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Brehm W, Aklin B, Yamashita T, Rieser F, Trüb T, Jakob RP, et al. Repair of superficial osteochondral defects with an autologous scaffold-free cartilage construct in a caprine model: implantation method and short-term results. Osteoarthritis Cartilage. 2006;14(12):1214-26. doi: 10.1016/j.joca.2006.05.002 [DOI] [PubMed] [Google Scholar]
- 48. Vikingsson L, Sancho-Tello M, Ruiz-Saurí A, Díaz SM, Gómez-Tejedor JA, Ferrer GG, et al. Implantation of a polycaprolactone scaffold with subchondral bone anchoring ameliorates nodules formation and other tissue alterations. Int J Artif Organs. 2015;38(12):659-66. doi: 10.5301/ijao.5000457 [DOI] [PubMed] [Google Scholar]
- 49. Lu Y, Dhanaraj S, Wang Z, Bradley DM, Bowman SM, Cole BJ, et al. Minced cartilage without cell culture serves as an effective intraoperative cell source for cartilage repair. J Orthop Res. 2006;24(6):1261-70. doi: 10.1002/jor.20135 [DOI] [PubMed] [Google Scholar]
- 50. Frisbie DD, Lu Y, Kawcak CE, DiCarlo EF, Binette F, McIlwraith CW. In vivo evaluation of autologous cartilage fragment-loaded scaffolds implanted into equine articular defects and compared with autologous chondrocyte implantation. Am J Sports Med. 2009;37(1 Suppl):71S-80S. doi: 10.1177/0363546509348478 [DOI] [PubMed] [Google Scholar]
- 51. Kim IL, Pfeifer CG, Fisher MB, Saxena V, Meloni GR, Kwon MY, et al. Fibrous scaffolds with varied fiber chemistry and growth factor delivery promote repair in a porcine cartilage defect model. Tissue Eng Part A. 2015;21(21-22):2680-90. doi: 10.1089/ten.tea.2015.0150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Erggelet C, Vavken P. Microfracture for the treatment of cartilage defects in the knee joint—a golden standard? J Clin Orthop trauma. 2016;7(3):145-52. doi: 10.1016/j.jcot.2016.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Moutos FT, Guilak F. Functional properties of cell-seeded three-dimensionally woven poly(ε-caprolactone) scaffolds for cartilage tissue engineering. Tissue Eng Part A. 2009;16(4):1291-301. doi: 10.1089/ten.tea.2009.0480 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental for Resorbable Pins to Enhance Scaffold Retention in a Porcine Chondral Defect Model by Jay M. Patel, Mackenzie L. Sennett, Anthony R. Martin, Kamiel S. Saleh, Michael R. Eby, Blair S. Ashley, Liane M. Miller, George R. Dodge, Jason A. Burdick, James L. Carey and Robert L. Mauck in CARTILAGE