Figure EV5. SigX impact on phage tolerance is Dlt‐mediated.
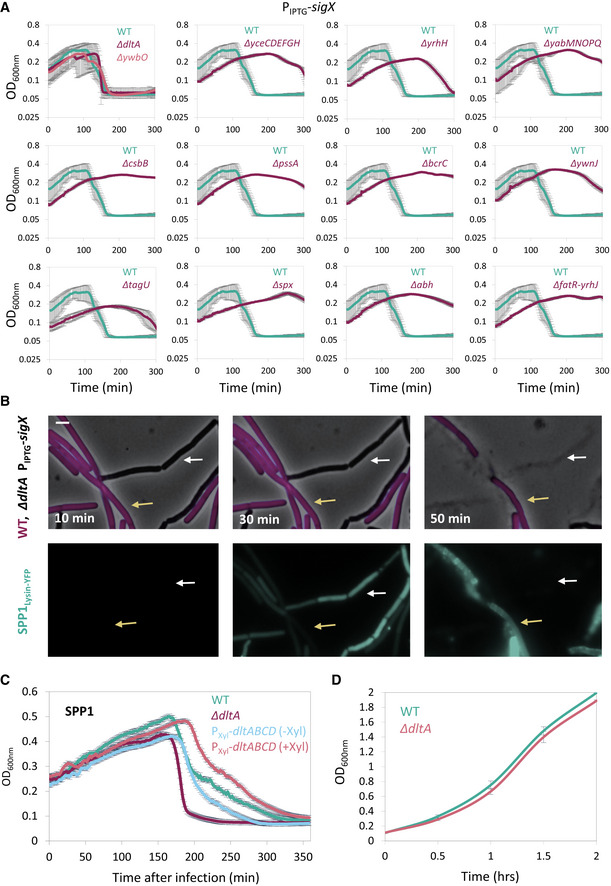
- Bacterial strains harboring PIPTG ‐sigX as well as the indicated gene deletions were grown in the presence of IPTG. At t = 60 min, cells were infected with SPP1 at low (phages:bacteria 1:20) MOI, and OD600nm was followed at 2‐min intervals. PY79 (WT) was infected in parallel for comparison. Knockout of ywbO and the dlt operon (ΔdltA) largely abolished the tolerance to phage infection conferred by SigX over‐expression. Shown is a representative experiment out of 3 independent biological repeats, with average values and SD of 3 technical repeats.
- ET42 (ΔdltA, PIPTG ‐sigX) cells were grown in the presence of IPTG and mixed with BDR2637 (Pveg ‐mCherry) (WT, purple) cells. The mixture was infected with SPP1‐lysin‐yfp at 5:1 (phages:bacteria) MOI, placed on an IPTG‐containing agarose pad, and followed by time‐lapse fluorescence microscopy. Shown are overlay images of phase contrast (gray) and signal from mCherry‐labeled cells (purple) (upper panels), and the corresponding signal from Lysin‐SPP1‐YFP (green) (lower panels), captured at the indicated time points. Yellow arrows denote infected WT cells, whereas white arrows highlight infected ET42 cells that lysed rapidly. Scale bar, 1 μm.
- PY79 (WT), ET41 (∆dltA), and ET72 (PXyl‐dltABCD) cells, grown with or without xylose as indicated, were infected with SPP1 at low (1:20) MOI, and OD600nm was followed at 2‐min intervals. Shown is a representative experiment out of 2 biological repeats, and the average values and SD of 4 technical repeats.
- PY79 (WT) and ET41 (ΔdltA) cells were grown in LB liquid medium, and OD600nm was followed. Shown are average values and SD of 3 biological repeats.
Source data are available online for this figure.
