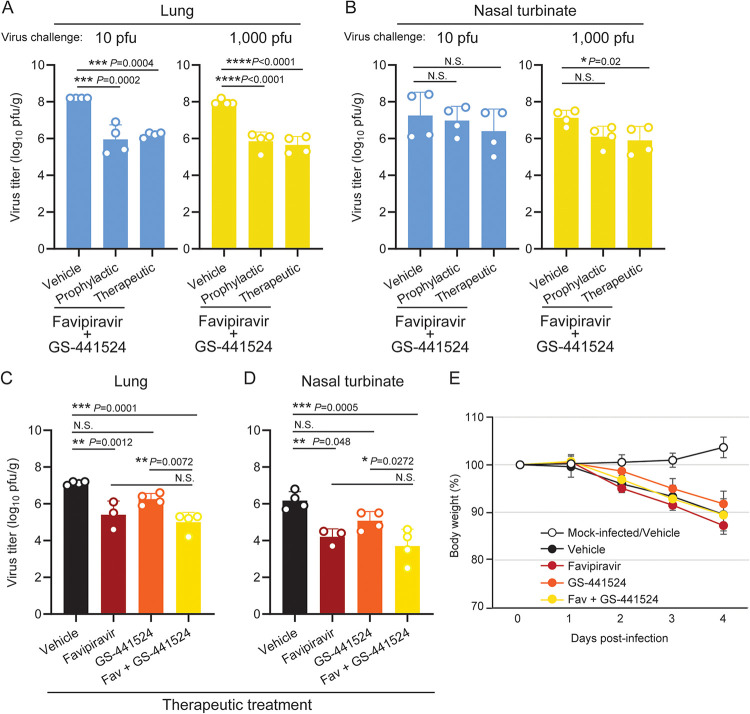
FIG 3.
Inhibitory effects of combination of GS-441524 and favipiravir on SARS-CoV-2 replication in the lungs and nasal turbinate of hamsters. (A, B) Each group of hamsters (n = 4) was administered vehicle control or the combination of favipiravir (300 mg/kg) and GS-441524 (25 mg/kg) as a prophylactic regimen (days –1 to 3; twice/day) or a therapeutic regimen (days 1 to 3; twice/day). The hamsters were intranasally inoculated with 10 or 1,000 PFU of SARS-CoV-2 (day 0). Infectious virus titers (PFU/g) in the lungs (panel A) and nasal turbinate (panel B) were examined by performing plaque assays. (C to E) Each group of hamsters (n = 3 for favipiravir treatment; n = 4 for other groups) was mock-infected or infected with 1,000 PFU of SARS-CoV-2 (day 0), and was administered vehicle control, favipiravir (300 mg/kg) GS-441524 (25 mg/kg), or a combination of both drugs as a therapeutic regimen (days 1 to 3; twice/day). Infectious virus titers (PFU/g) in the lungs (panel C) and nasal turbinate (panel D) were examined by performing plaque assays. Body weight change was monitored for 4 days (panel E; mean ± SD). Dots and bars in panels A to D show the value for each animal and the average in each group, respectively (mean ± SD). *, P < 0.05; NS, not significant; determined by a one-way analysis of variance (ANOVA) and corrected for multi-group comparison using Dunnett’s test.