Abstract
Background
Few studies have reported a double-step follow-up of patients after hospitalization for COVID-19.
Objectives
We designed an observational double-step follow-up study with a clinical, functional, and radiological evaluation at 2 and 6 months after COVID-19. The primary outcome was to describe symptoms, spirometry, and 6-minute walking test (6MWT) at 2 and 6 months. Secondary outcomes were to identify if the lowest PaO<sub>2</sub>/FiO<sub>2</sub> during hospitalization is related with functional and radiological evolution and to assess the correlation between radiological and functional abnormalities at 6 months.
Methods
Symptoms, spirometry, and 6MWT were assessed at 2 and 6 months; arterial blood gas, chest x-ray, and lung ultrasound were performed at 2 months; body plethysmography, diffusing capacity for carbon monoxide (DLCO), and CT scan were performed at 6 months.
Results
Sixty-four per cent and 42% of patients reported at least one symptom at 2 and 6 months, respectively. The most common 6-month functional alteration was DLCO impairment (57% of patients). An improvement of FEV1, FVC, and 6MWT was observed between 2 and 6 months (p < 0.001). Patients with PaO<sub>2</sub>/FiO<sub>2</sub> <200 during hospitalization performed worse at 6MWT at 2 and 6 months (p < 0.05) and reported more extended radiological abnormalities at 6 months (p < 0.001) compared with patients with PaO<sub>2</sub>/FiO<sub>2</sub>>200. At 6 months, more extended radiological abnormalities were related with worse 6MWT, DLCO, and total lung capacity (p < 0.05).
Discussion
DLCO and 6MWT impairment seem to be the functional hallmark of COVID-19 and are related with the severity of acute pneumonia. At 6 months, radiological abnormalities were related to functional impairment.
Keywords: COVID-19, Pneumonia, Respiratory infection, Follow-up, Sequelae.
Introduction
Few studies have addressed the problem of clinical and functional aftermath in patients after hospitalization for COVID-19 Pneumonia [1, 2, 3, 4, 5, 6, 7, 8, 9, 10]. Most of these studies have assessed only one aspect of lung sequelae, such as symptoms [1], pulmonary function tests (PFTs) [2, 3, 4], and high-resolution computed tomography (HRCT) [5, 6]. Other studies performed a comprehensive evaluation, reporting both clinical and functional/radiological aspects [7, 8, 9, 10]. Recently, a comprehensive large Chinese monocentric cohort study showed that 6 months after the acute disease 76% of patients reported at least one symptom (especially fatigue, muscle weakness, and sleep difficulties), up to 56% of patients had diffusion dysfunction and that the most common HRCT pattern was pulmonary interstitial changes (ground-glass opacities [GGOs] and irregular lines) [7]. In a monocentric French study [8] about previously hospitalized patients, 51% of 478 subjects reported during a telephone interview at least one symptom (especially fatigue, cognitive impairment, or dyspnoea) that was not present before COVID-19; new onset dyspnoea was reported in 16% of patients. One hundred seventy-seven patients were assessed in the outpatient clinic 4 months after discharge: HRCT abnormalities and diffusing capacity for carbon monoxide (DLCO) impairment were more frequent in patient who underwent intubation during hospitalization. In the same series, left ventricular ejection fraction was >50% in 90% of the patients, and in none it was <40%. All the subjects with an ejection fraction <50% were former intensive care unit patients [8]. An Italian study reported that 58% and 44% of patients showed alterations of DLCO and chest X-ray (CXR), respectively [10].
Very few studies assessed the natural history of the disease by a double-step follow-up after withdrawing any therapy of the acute phase. An observational study about corticosteroid treatment was performed in England in patients with persistent HRCT abnormalities and PFTs dysfunction 4 weeks after discharge and an improvement of symptoms, radiological signs, and lung function was shown after treatment [11]. Li et al. [12] reported spirometry at 2 and approximately 4 weeks from discharge, showing a progressive functional improvement in vital capacity, forced vital capacity (FVC), and forced expiratory volume in 1 s (FEV1) in 18 patients. Liu et al. [13] described the respiratory functional effects of a 6-week respiratory rehabilitation training in 72 patients by 2 evaluations: the first almost at 6 months from the onset of COVID-19 and the second after 6 weeks. An improvement of spirometry, DLCO, and 6-Minute Walking Distance (6MWD) was observed [13]. Indeed, only a guidance by British Thoracic Society (BTS) suggesting a different follow-up according to the severity of the acute pneumonia had already been published when this study was designed [14, 15]. We therefore planned a prospective observational monocentric follow-up study to assess the clinical, functional, and imaging evolution of the disease (2 months and 6 months after discharge) without any pharmacologic therapy and out of any rehabilitation program. Symptoms, spirometry, and 6MWT were assessed at 2 and 6 months; arterial blood gas (ABG), CXR, and lung ultrasound (LUS) were performed at 2 months; body plethysmography, DLCO, and HRCT were performed at 6 months. The primary outcome was to describe symptoms, spirometry, and exercise capacity (6MWT) at 2 and 6 months.
Secondary outcomes were:
To evaluate if the patients with more severe acute respiratory failure defined by impairment of gas exchange (lowest value of the ratio of arterial oxygen partial pressure [PaO2 in mmHg] to fractional inspired oxygen [FiO2], PaO2/FiO2 nadir) had worse functional (lung function impairment and 6MWT) and radiological sequelae (HRCT severity score) at 6 months [16].
To assess the correlation between radiological abnormalities (HRCT severity score) and functional impairment at 6 months.
Materials and Methods
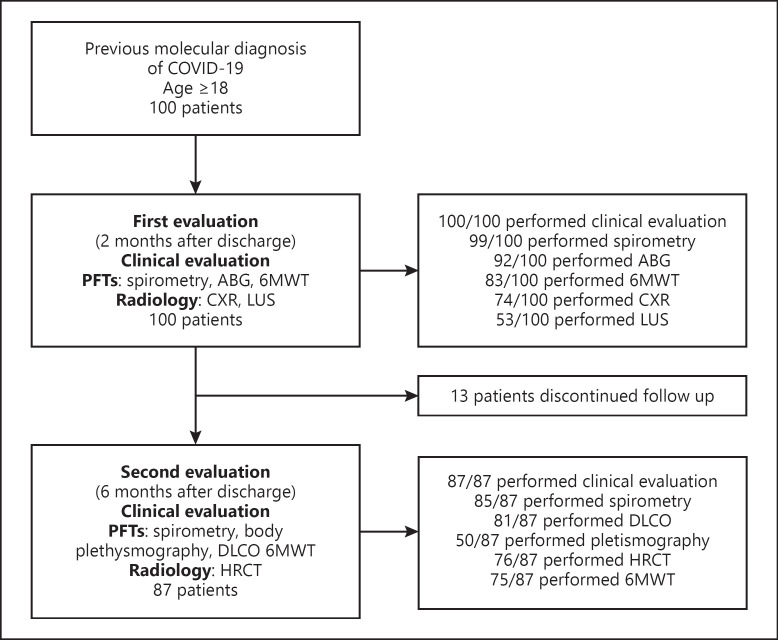
Inclusion criteria were a previous molecular diagnosis of COVID-19 (by reverse transcriptase-polymerase chain reaction on naso-pharyngeal swab at admission to hospital) and an age older than 18 years. Patients signed a written informed consent. No exclusion criteria were present. Patients included in the study were hospitalized during the first Italian epidemic wave (March 1, 2020–May 31, 2020) and were followed up at the Pulmonary and Respiratory Intensive Care Unit of Sant'Orsola-Malpighi hospital until November 30, 2020. Our 2 steps follow-up included: an “early” appointment within 2 months from discharge and a second one, within 6 months from discharge. All patients were seen face to face by 2 trained physicians. Figure 1 shows the flow chart of the study, with the number of patients that were present at each evaluation. The data regarding the acute phase (the interval between symptoms onset to discharge from hospital) were taken retrospectively from the hospital medical records and they are shown on Table 1.
Fig. 1.
Flow chart of the study.
Table 1.
Baseline characteristics of our study population
| Total | No support or standard oxygen | IMV, NIV, CPAP, and HFNC | p value | |
|---|---|---|---|---|
| Past medical history Patients, n | 100 | 66 | 34 | |
| Age, mean value (SD) | 60 (14) | 59 (15) | 64 (11) | 0.055 |
| Male | 55/100 | 34 (51%) | 21 (62%) | 0.334 |
| BMI, mean value (SD) | 26 (5) | 26 (5) | 26 (3) | 0.539 |
| Former smokers | 37/100 | 22 (33%) | 15 (44%) | 0.621 |
| Pack/years, mean value (SD) | 24 (18) | 22 (19) | 21 (19) | 0.413 |
| Pulmonary comorbidities (asthma, COPD, and ILD) | 20/100 | 15 (23%) | 5 (15%) | 0.347 |
| Cardiovascular comorbidities (hypertension, CHD, and diabetes) | 50/100 | 25 (38%) | 25 (74%) | 0.001 |
| Previous malignancy | 6/100 | 4 (6%) | 2 (6%) | 0.987 |
| Acute phase of COVID-19 | ||||
| Time to hospital admission mean value (SD) | 7 (4) | 7 (4) | 6 (4) | 0.257 |
| HLOS, mean value (SD) | 22 (14) | 15 (9) | 34 (15) | 0.000 |
| HRCT severity score, mean value (SD) | 13 (6) | 10 (4) | 17 (5) | 0.000 |
| ABG, mean value, mm Hg (SD) | ||||
| PaO2/FiO2 at hospital admission | 320 (91) | 342 (65) | 276 (118) | 0.001 |
| PaO2/FiO2 nadir | 230 (108) | 297 (66) | 108 (42) | 0.000 |
| PaO2/FiO2 at hospital discharge | 361 (66) | 377 (66) | 334 (59) | 0.023 |
| Blood test, mean value (SD) | ||||
| CRP (at hospital admission), mg/dL | 6 (6) | 5 (6) | 7 (6) | 0.092 |
| LDH (at hospital admission), U/L | 287 (136) | 246 (79) | 357 (180) | 0.000 |
| IL-6 (max), pg/mL | 64 (171) | 50 (172) | 87 (170) | 0.357 |
| D dimer (max), mg/L | 3 (7) | 2 (4) | 5 (10) | 0.162 |
| Therapy | ||||
| Hydroxychloroquine | 92/100 | 60 (91%) | 32 (94%) | 0.013 |
| Azithromycin | 55/100 | 35 (53%) | 20 (59%) | 0.020 |
| Steroids | 61/100 | 34 (52%) | 27 (79%) | 0.000 |
| Antivirals | 23/100 | 13 (19%) | 10 (29%) | 0.014 |
| Tocilizumab | 46/100 | 17 (26%) | 29 (85%) | 0.000 |
Bold p values are significant. HFNC, high-flow nasal cannula; NIV, non-invasive ventilation; CPAP, continuous positive airway pressure; IMV, invasive mechanical ventilation; SD, standard deviation; BMI, body mass index; CHD, chronic heart disease; CRP, C-reactive protein at hospital admission (first available value); LDH, lactate dehydrogenase at hospital admission (first available value); IL-6, interleukin 6; Max, highest value during hospitalization.
At 2 months we recorded PFTs (spirometry), 6MWT, ABG, CXR, LUS, and symptoms. To do that, we have pre-identified 12 symptoms on a checklist, and the patients reported or not their presence (cough, dyspnoea, chest pain, fatigue, fever, muscle weakness, tachycardia, throat pain, nausea, diarrhoea, balance disorder, and stomach ache). Indeed, they were free to report any other symptoms. At 6 months, we recorded symptoms, PFTs (spirometry, body plethysmography, and DLCO), HRCT, and 6MWT. ABG was performed only at 2 months as we expected a normalization of PaO2/FiO2 since its value at discharge was already above 300 mm Hg.
6MWT was performed according to actual guidelines, including the minimum number of tests [17]; we recorded 6MWD, the percent predicted value (calculated according to Gibbons et al. [18]), the lowest saturation during 6MWT (lowest oxygen saturation, SpO2%) and the highest heart rate during 6MWT (highest HR), the difference between the initial SpO2 value and the lowest SpO2 value during the 6MWT (6MWT desaturation %, e.g., from 98 to 95% desaturation is 3%), and the distance saturation product (DSP: 6MWD × lowest SaO2 during the 6MWT [19]).
Concerning PFTs, we defined obstructive pattern by a FEV1/FVC ratio less than 70, we defined possible restrictive pattern by a FVC less than 80% of the predicted value (since it was impossible to perform body plethysmography at 2 months), and we defined a possible mixed pattern if the FEV1/FVC ratio was less than 70 and FVC was less than 80% predicted value, according to Johnson et al. [20].
CXR was performed at 2 months in 74 patients as 26 refused another radiological study as many were performed in hospital during COVID-19 acute phase, and a HRCT was already planned within 4 months. CXR was classified from a team of 3 expert radiologists as negative, presence of opacities, interstitial thickening, or peripheral fibrotic linear images. A semi-quantitative scoring system called Brixia CXR score, that assigns a score (from 0 to 3) to each of 6 zones on frontal chest projection, was used to perform a radiological quantification of lung abnormalities by 3 radiologists [21, 22].
The LUS examinations were all performed with a My LabTM Eight machine (Esaote, Genova, Italy) by 2 experienced operators. The convex probe (3–5 MHz) was used, with the widest acoustic window and maximum depth of 10 cm, with the focus on the pleural line. Mechanical Index was <0.9. The examination was performed in the sitting, lateral, or supine position. Several scoring systems are reported in literature to evaluate COVID-19 pneumonia during acute phase [23, 24]. According to the systematic protocol of scanning already described in literature for each patient 12 areas have been explored and reported in the LUS score (LUSs) [23]. The areas have been registered as right and left lung areas (R and L) and divided by using the anterior and posterior axillary lines resulting in 3 areas per hemithorax (anterior, lateral, and posterior), each of them splits in superior and inferior. Each area was examined in the sagittal and axial views. All the lung areas were explored, and for each one the score was defined with the semi-quantitative assessment of pulmonary aeration loss: 0: normal lung (A lines); 1: non-coalescent B lines (B lines occupying less than 50% of the intercostal space in the transversal plane); 2: coalescent B lines (B lines occupying more than 50% of the intercostal space); 3: consolidation >1 cm. The sum of the 12 different lung areas represents the LUSs and it can variate between 0 and 36. The presence of irregular thickening of the pleural line was reported as “irregular pleural line pattern (IPP)” and could be observed both alone and together with B lines. We specifically reported IPP because pleural line irregularity is one of the most common sonographic findings of COVID-19 patients [25]. The whole examination lasted approximately 10 min. As suggested by the international evidence-based recommendations for point-of-care LUS, a positive finding of interstitial pulmonary syndrome is the presence of 2 or more positive regions bilaterally, with a LUSs cut-off ≥2 [26].
HRCT severity score was visually and independently calculated by 3 different radiologists according to Lessmann et al. [16]. A score 0–5 was assigned to each lobe: 0 = no lobe involvement; 1 = involvement <5%; score 2 = involvement >5% and <25%; score 3 = involvement >25% and <50%; score 4 = involvement >50% and <75% and score 5 = involvement >75%; so that the range of total score was 0–25. Discrepancies were solved by mutual agreement of the 3 radiologists after reviewing HRCT. We excluded from the radiological analysis patients with prior interstitial lung disease (ILD) because it could be difficult to differentiate between prior ILD (maybe worsened after COVID-19) and COVID-19 sequelae.
At 2 months steroids were withdrawn in patients still on treatment for organizing pneumonia-like lesions as the pattern was felt to be stabilized because the triggering viral infection had cleared since at least 30 days and because of the clinical improvement with no ongoing driver of inflammation. Standard inhalation therapy was given to patients with a diagnosis of chronic obstructive pulmonary disease (COPD). No patient underwent other respiratory treatments or a formal rehabilitation program.
Statistical analysis was made using IBM SPSS Statistics version 21.0. Differences between variables were analysed using one-way analysis of variance, when appropriate. Relationship between variables was studied using Spearman's correlation, p < 0.05 was considered statistically significant.
Results
Patients
One hundred consecutive patients with COVID-19 were studied, including the full range of pneumonia severity. 10 patients underwent invasive mechanical ventilation, 24 were supported either with non-invasive ventilation, continuous positive airway pressure, or high-flow nasal cannula, whilst 66 patients received standard oxygen therapy or no support at all. Demographic and clinical characteristics of patients during hospitalization divided in 2 groups according to the highest respiratory support received are shown in Table 1. The mean age was 60 years; 37% of patients were former smokers (24 pack/years of average), 50% had a cardiovascular comorbidity (hypertension 44%, chronic heart disease 11%, and diabetes 14%) and 22% had a respiratory comorbidity (COPD 9%, asthma 10%, ILD 3%); 40% were obese (body mass index >29) and 40% overweight (25< body mass index <30). Cardiovascular comorbidities were significantly more prevalent in the group with higher ventilatory support (p < 0.001). PaO2/FiO2 nadir and HRCT extension score were worse in the group of patients with higher ventilatory support (p < 0.001). Lactate dehydrogenase at hospital admission was higher in the group with higher ventilatory support (p < 0.001). More patients of the higher ventilatory support group received therapies (hydroxychloroquine, azithromycin, steroids, antivirals, and tocilizumab) compared to the lower ventilatory support group. Clinical, functional, and radiological characteristics of patients at 2 and 6 months are shown in Table 2.
Table 2.
Follow-up characteristics of our study population
| Follow-up: symptoms and physical examination | First evaluation (2 months after COVID-19) |
Second evaluation (6 months after COVID-19) |
Difference | p value |
|---|---|---|---|---|
| Dyspnoea (mMRC scale) | ||||
| 0 | 56/100 (56%) | 57/87 (65%) | +9% | |
| 1 | 32/100 (32%) | 26/87 (30%) | −2% | 0.017 |
| 2 | 12/100 (12%) | 4/87 (5%) | − 7% | |
| 3–4 | 0/100 (0%) | 0/87 (0%) | 0% | |
| Other symptoms | ||||
| Symptomatic patients | 64/100 (64%) | 48/87 (42%) | −22% | 0.925 |
| Fatigue | 13/100 (13%) | 12/87 (10%) | −3% | 0.832 |
| Muscular weakness | 8/100 (8%) | 6/87 (5%) | −3% | 1.000 |
| Cough | 10/100 (10%) | 7/87 (6%) | −4% | 0.623 |
| Chest pain | 6/100 (6%) | 5/87 (4%) | −2% | 1.000 |
| Tachycardia | 2/100 (2%) | 3/87 (3%) | + 1% | 0.653 |
| Stomach-ache | 6/100 (6%) | 6/87 (5%) | −1% | 1.000 |
| Throat pain | 4/100 (4%) | 6/87 (5%) | + 1% | 0.519 |
| Balance disorder | 1/100 (1%) | 0/87 (0%) | −1% | 0.319 |
| Nausea or diarrhoea | 2/100 (2%) | 3/87 (3%) | + 1% | 0.672 |
| Persistent-low grade fever | 3/100 (3%) | 1/87 (1%) | −2% | 0.315 |
| SpO2, mean value % (SD) | 97 (1) | 97 (1) | +0% | 0.706 |
| Altered pulmonary physical examination | 17/100 (17%) | 11/84 (13%) | −5% | 0.445 |
|
| ||||
| Follow-up: PFT | ||||
| PFT (pattern) | ||||
| Normal pattern | 76/99 (76%) | 73/85 (86%) | +10% | 0.009 |
| Obstructive pattern | 15/99 (15%) | 7/85 (8%) | −7% | 0.261 |
| Possible restrictive pattern | 8/99 (8%) | 3/85 (4%) | −4% | 0.394 |
| Possible mixed pattern | 1/99 (1%) | 2/85 (2%) | +1% | 0.499 |
| PFT | ||||
| FEV1 (raw data), mean value L/s (SD) | 2.85 (0.88) | 2.92 (0.84) | +0.07 L | <0.0001 |
| FEV1 (% predicted), mean value (SD) | 104 (19) | 107 (18) | +3% | 0.244 |
| Patients with FEV1 <80% predicted | 10/99 (10%) | 5/85 (6%) | −4% | 0.289 |
| FVC (raw data), mean value L (SD) | 3.56 (1.14) | 3.66 (1.00) | +0.10 L | <0.0001 |
| FVC (% predicted), mean value (SD) | 107 (20) | 109 (18) | +2% | 0.480 |
| FEV1/FVC, mean value (SD) | 79 (9) | 78 (7) | −1 | 0.778 |
| TLC (raw data), mean value L (SD) | NA | 5.49 (1.26) | NA | |
| TLC (% predicted) mean Value (SD) | NA | 95 (14) | NA | |
| DLCO | ||||
| % predicted, mean value, (SD) | NA | 75 (18) | NA | |
| Normal | NA | 35/81 (43%) | NA | |
| Mildly reduced (79%–60%) | NA | 29/81 (36%) | NA | |
| Moderately reduced (59%–40°%) | N.A | 16/81 (20%) | NA | |
| Severely reduced (below 40%) | N.A | 1/81 (1%) | NA | |
| ABG | ||||
| PaO2/FiO2, mean value mm Hg (SD) | 444 (49) | NA | NA | |
| 6MWT | ||||
| 6MWD, mean value m (SD) | 418 (117) | 489 (141) | +71 m | <0.0001 |
| 6MWD% predicted, mean value (SD) | 63 (17) | 73 (22) | +10% | <0.0001 |
| Patients with 6MWD <80% predicted | 70/83 (84%) | 48/75 (64%) | −20% | 0.002 |
| Lowest SpO2 %, mean value (SD) | 94 (4) | 95 (3) | +1% | 0.178 |
| DSP, mean value (SD) | 396 (115) | 466 (137) | +70 m% | <0.0001 |
| Highest heart rate, mean value bpm (SD) | 104 (21) | 107 (20) | +3 bpm | 1.000 |
| 6MWT desaturation ≥3% (i.e., from 96% to 92%) | 37/83 (45%) | 26/75 (34%) | −11% | <0.0001 |
| Follow-up: radiology | ||||
| CXR | ||||
| Negative | 34/74 (46%) | NA | NA | |
| Interstitial thickening | 25/74 (34%) | NA | NA | |
| Parenchymal opacities | 12/74 (16%) | NA | NA | |
| Fibrotic striae | 20/74 (27%) | NA | NA | |
| Brixia CXR score | 3 (3) | NA | NA | |
| LUS | ||||
| LUS score | 7 (6) | NA | NA | |
| HRCT | ||||
| Severity score, mean value (SD) | NA | 6 (6) | NA | |
| Negative | NA | 19/74 (26%) | NA | |
| Ground glass | NA | 42/74 (57%) | NA | |
| Crazy paving | NA | 0/74 (0%) | NA | |
| Consolidation | NA | 0/74 (0%) | NA | |
| Lines | NA | 13/74 (18%) | NA | |
Bold p values are significant. mMRC, Modified British Medical Research Council Questionnaire; SpO2, oxygen saturation; NA, not available.
Symptoms
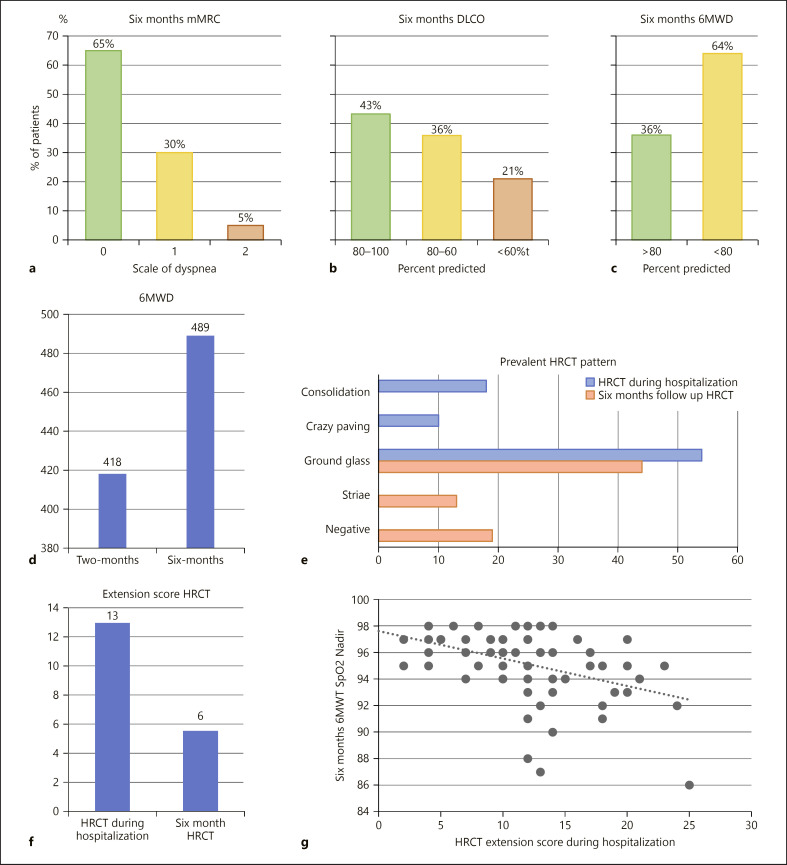
Symptoms reported are shown in Table 2. At 2 months, 64% of patients were still symptomatic. Exertional dyspnoea (44%), fatigue (13%), and cough (10%) were the most prevalent symptoms, whilst other symptoms were reported by less than 10% of patients. At 6 months, the total rate of patients reporting at least one symptom decreased (42%, −22%). The variation of dyspnoea assessed by Modified British Medical Research Council Questionnaire (mMRC) from 2 to 6 months was statistically significant (Table 2; Fig. 2a). Table 3 shows a significant correlation between symptoms at 6 months and time to hospital admission (p < 0.05).
Fig. 2.
a6-months severity mMRC scale of dyspnoea.b6-months–severity DLCO (normal, mild, and moderate impairment).c6-months 6MWD over/under 80 per cent predicted.dComparison between 2-months 6MWD and 6-months 6MWD.eCharacteristics of HRCT during hospitalization and at 6 months.fComparison between extension score HRCT during hospitalization and 6-months HRCT.gCorrelation between extension score HRCT during hospitalization and 6-months 6MWT SpO2 Nadir. mMRC, Modified British Medical Research Council Questionnaire.
Table 3.
Correlation between parameters during hospitalization and clinical, functional, and radiological parameters 6 months after COVID-19
| Acute phase |
6-month evaluation |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HRCT score during H |
symptoms, n |
6MWT desaturation, % |
6MWD |
DSP |
6MWD % predicted |
FEV1% predicted |
DLCO % predicted |
6-month HRCT score |
||||||||||
| cc |
p value |
cc |
p value |
cc |
p value |
cc |
p value |
cc |
p value |
cc |
p value |
cc |
p value |
cc |
p value |
cc | p value | |
| Acute phase Age | 0.200 | 0.68 | −0.020 | 0.843 | −0.171 | 0.140 | −0.332 | 0.003 | −0.355 | 0.002 | −0.128 | 0.267 | 0.142 | 0.193 | −0.323 | 0.003 | 0.414 | 0.000 |
| CRP (at hospital admission) | 0.371 | 0.001 | −0.003 | 0.980 | −0.080 | 0.521 | −0.253 | 0.039 | −0.268 | 0.028 | −0.162 | 0.188 | 0.105 | 0.366 | 0.042 | 0.725 | 0.425 | 0.000 |
| IL–6 (max) | 0.377 | 0.001 | 0.112 | 0.334 | −0.126 | 0.338 | −0.174 | 0.184 | −0.197 | 0.130 | −0.085 | 0.514 | 0.104 | 0.395 | −0.220 | 0.073 | 0.192 | 0.142 |
| D-dimer (max) | 0.380 | 0.004 | 0.041 | 0.747 | −0.157 | 0.268 | 0.006 | 0.968 | −0.017 | 0.907 | 0.064 | 0.650 | 0.112 | 0.408 | −0.134 | 0.323 | 0.416 | 0.003 |
| Time to hospital admission | 0.099 | 0.391 | 0.302 | 0.005 | −0.023 | 0.856 | −0.008 | 0.951 | −0.024 | 0.845 | 0.074 | 0.547 | 0.042 | 0.723 | 0.102 | 0.394 | 0.118 | 0.346 |
| HLOS | 0.545 | 0.000 | −0.093 | 0.392 | −0.377 | 0.002 | −0.218 | 0.077 | −0.251 | 0.040 | −0.074 | 0.547 | 0.274 | 0.018 | −0.250 | 0.034 | 0.574 | 0.000 |
| PaO2/FiO2 at hospital admission | −0.264 | 0.020 | −0.091 | 0.406 | 0.198 | 0.110 | 0.301 | 0.014 | 0.309 | 0.012 | 0.267 | 0.029 | −0.065 | 0.577 | 0.112 | 0.349 | −0.200 | 0.105 |
| PaO2/FiO2 nadir | −0.641 | 0.000 | 0.039 | 0.722 | 0.231 | 0.060 | 0.126 | 0.310 | 0.147 | 0.235 | 0.075 | 0.544 | −0.178 | 0.124 | 0.234 | 0.048 | −0.453 | 0.000 |
| PaO2/FiO2 at hospital discharge | −0.313 | 0.010 | −0.017 | 0.888 | 0.232 | 0.072 | 0.208 | 0.107 | 0.208 | 0.108 | 0.213 | 0.099 | −0.041 | 0.739 | 0.206 | 0.105 | −0.276 | 0.036 |
| HRCT score during H | 1.000 | −0.088 | 0.428 | −0.274 | 0.029 | −0.211 | 0.094 | −0.242 | 0.054 | −0.202 | 0.107 | 0.143 | 0.227 | −0.209 | 0.084 | 0.561 | 0.000 | |
Bold values are significant. Cc, correlation coefficient; CRP, C-reactive protein at hospital admission (first available value); IL-6, interleukin 6; Max, highest value during hospitalization; PaO2/FiO2, ratio of arterial oxygen partial pressure (PaO2 in mm Hg) to fractional inspired oxygen (FiO2); H, hospitalization.
Pulmonary Function Tests
Mean results of spirometry, body plethysmography, and DLCO are shown in Table 2. At 2 months, spirometry was performed in 99 patients and showed a normal pattern in 76% of cases versus 86% at 6 months, when it was performed in 87 subjects. A statistically significant improvement was observed between 2 and 6 months for FEV1 and FVC (p < 0.001). Patients with a normal pattern significantly increase from 2 to 6 months (+10%, p < 0.05). At 6 months 43% of the patients had a normal value of DLCO, 36% showed a mild reduction, and 21% a moderate/severe impairment (shown in Fig. 2b). The mean PaO2/FiO2 value at 2 months was 444 mm Hg.
Six-Minute Walking Test .6MWT distance and variables are reported in Table 2. 83 patients performed the 6MWT at 2 months and 75 at 6 months. Patients who did not perform the test had orthopaedic abnormalities, were unable to walk unassisted after COVID-19 hospitalization or refused it. Only the patients who performed 6MWT at 2 months were asked to perform it again at 6 months. The mean distance (6MWD) was 418 m (63% predicted) at 2 months and 489 m (73% predicted) at 6 months (p < 0.001) with 84% of patients walking <80% of predicted at 2 months and 64% at 6 months (shown in Fig. 2c, d) (p < 0.05, Table 2). At 2 and 6 months, 45% and 34% experienced a desaturation larger than 3% (p < 0.001), respectively. DSP was 395 at 2 months versus 466 at 6 months (p < 0.001).
Table 3 shows a significant correlation between 6MWT desaturation and DSP and hospital length of stay (HLOS), between 6MWD and DSP and PaO2/FiO2 at admission, and between 6MWT desaturation and HRCT extension score during hospitalization.
Imaging
Data concerning CXR, LUS, and HRCT are shown in Table 2.
Chest X-ray. Two months after hospital discharge, we performed CXR in 74 patients: 46% of them were normal; areas of parenchymal opacities were present in 16% of patients, interstitial thickening in 34%, and mostly peripheral fibrotic linear images in 27% of cases. The lowest Brixia Score 2 months after acute phase of COVID-19 was 0 (18 patients) and the highest score was 8 (4 patients), with a mean score of 3 (±3).
Lung Ultrasound . LUS was performed 2 months after discharge in 53 patients. The lowest LUS score 2 months after acute phase of COVID-19 was 0 (20 patients) and the highest score was 25 (1 patients), with a mean score of 7 (±6). A small pleural effusion was found in 12 patients (22%), often localized in posterior-basal regions (R6-L6). Irregular pleural line pattern was reported in 50 patients (94%), whilst irregular pleural pattern in absence of B lines was found in 20 patients (38%). Interstitial pulmonary syndrome (the presence of 2 or more positive regions bilaterally, with a LUSs cut off ≥2) was diagnosed at 2 months in 31 patients (59%).
High-Resolution Computed Tomography. Eighty-four patients performed HRCT during the acute phase (hospitalization) and 76 patients performed HRCT after 6 months. HRCT at 6 months was compared to HRCT during hospitalization using the HRCT severity score: there was a reduction in extension of the disease (mean score decreased from 13/25 to 6/25 [p < 0.001] as shown in Table 3.) with a shift from consolidation and crazy paving during hospitalization to fibrotic lines and normal lung at 6 months (shown in Fig. 2e, f). Furthermore, the density of GGOs was decreasing at 6 months as if they were fading away.
Correlation between Severity of Pneumonia and Sequelae
Table 4 shows the clinical and radiological features of the patients in the acute phase, at 2 and at 6 months divided according to the lowest PaO2/FiO2 (PaO2/FiO2 nadir). Patients with PaO2/FiO2 <200 during hospitalization performed significantly worse at 6MWT (p < 0.05 for all the recorded parameters) 2 months after discharge. At 6 months, there is no longer a significant difference in 6MWD and DSP, whilst a significant difference was still present for lowest SpO2 and 6MWT desaturation %. A significant difference between these 2 groups in HRCT severity score was present both during hospitalization and at 6 months.
Table 4.
Acute phase, first, and second evaluation parameters divided by PaO2/FiO2 nadir
| PaO2/FiO2 nadir >200 mm Hg, mean value (SD) 51 |
PaO2/FiO2 nadir ≤200 mm Hg, mean value (SD) 37 |
p value NA | |
|---|---|---|---|
| Patients, n | 51 | 37 | NA |
|
| |||
| Hospitalization | |||
| Time to hospital admission | 7 (5) | 6 (4) | 0.335 |
| HLOS | 14 (9) | 33 (15) | 0.000 |
| PaO2/FiO2 at admission | 354 (53) | 270 (115) | 0.000 |
| PaO2/FiO2 at discharge | 381 (67) | 334 (57) | 0.002 |
| CRP (at hospital admission) | 4 (5) | 8 (6) | 0.001 |
| IL-6 (max) | 20 (21) | 119 (250) | 0.014 |
| D-dimer (max) | 1 (2) | 5 (10) | 0.037 |
| HRCT severity score | 9 (5) | 17 (5) | 0.000 |
|
| |||
| First evaluation (2 months) | |||
| 6MWT lowest SpO2 % | 96 (2) | 93 (6) | 0.009 |
| 6MWT desaturation % | 2 (29) | 4 (6) | 0.012 |
| 6MWD | 446 (103) | 390 (113) | 0.033 |
| DSP | 426 (103) | 363 (112) | 0.013 |
| 6MWD % predicted | 67 (16) | 59 (14) | 0.033 |
| PaO2/FiO2 | 444 (78) | 433 (48) | 0.191 |
| FEV1 % predicted | 101 (21) | 105 (23) | 0.338 |
| FVC % predicted | 107 (26) | 107 (23) | 0.965 |
|
| |||
| Second evaluation (6 months) | |||
| 6MWT lowest SpO2 % | 96 (1) | 94 (3) | 0.000 |
| 6MWT desaturation % | 2 (1) | 3 (2) | 0.002 |
| 6MWD | 513 (140) | 454 (139) | 0.087 |
| DSP | 494 (134) | 428 (135) | 0.051 |
| 6MWD % predicted | 77 (21) | 70 (20) | 0.123 |
| FEV1 % predicted | 105 (19) | 111 (17) | 0.151 |
| FVC % predicted | 108 (21) | 111 (17) | 0.533 |
| DLCO % predicted | 79 (16) | 71 (21) | 0.081 |
| HRCT severity score | 3 (4) | 9 (6) | 0.000 |
Bold p values are significant. SD, standard deviation; CRP, C-reactive protein at the time of hospital admission (first available value); IL-6, interleukin 6; Max, highest value during hospitalization; SpO2, oxygen saturation.
As shown in Table 3, several correlations were found between different physiological and radiological variables. DLCO at 6 months was positively correlated to PaO2/FiO2 nadir during hospitalization and inversely to HLOS. DSP during 6MWT was inversely correlated to HLOS and CRP. 6MWT desaturation % was inversely related to HRCT severity score during hospitalization (shown in Fig. 2g). The overall symptoms were not correlated with any parameters except for the time lag between the onset of symptoms and hospitalization (p < 0.05).
Correlation between HRCT and Lung Function at 6 Months
Table 5 shows some significant correlations between 6 months HRCT severity score and 6MWT, DLCO and body plethysmography at 6 months. There was a significant inverse correlation between SpO2 measured at clinical evaluation and HRCT severity score at 6 months (p < 0.05). Concerning 6MWT, a higher HRCT severity score is associated with a shorter distance (6MWD), a lower SpO2 during 6MWT, and consequently a lower DSP (p < 0.05). There was a significant inverse correlation between DLCO and total lung capacity (TLC) and HRCT severity score at 6 months (p < 0.05).
Table 5.
Correlation between clinical and functional and radiological parameters 6 months after COVID-19
| 6-month score HRCT | ||
|---|---|---|
| CC | p value | |
| 6-month evaluation | ||
| Symptoms (n) | −0.159 | 0.174 |
| SpO2 % | −0.295 | 0.015 |
| 6MWT lowest SpO2 % | −0.328 | 0.011 |
| 6MWT desaturation (%) | −0.220 | 0.094 |
| 6MWD | −0.288 | 0.027 |
| 6MWD % predicted | −0.162 | 0.216 |
| DSP | −0.313 | 0.016 |
| FEV1 % predicted | −0.022 | 0.862 |
| FVC % predicted | −0.156 | 0.208 |
| FEV1/FVC | 0.076 | 0.540 |
| DLCO % predicted | −0.269 | 0.033 |
| TLC % predicted | −0.352 | 0.021 |
Bold values are significant. CC, correlation coefficient; SpO2, oxygen saturation; TLC, total lung capacity.
Discussion
This is a real-life assessment of the clinical, functional, and radiological trajectory of recovery in patients hospitalized for COVID-19 pneumonia. We planned a double-step follow-up: the interim 2 months evaluation included symptoms, ABG and imaging (CXR and LUS) to rule out complications (e.g., new pulmonary infiltrates and pneumothorax) and a simple functional evaluation (spirometry and 6MWT) according to the staff availability in our centre at that time; the 6-month visit was a comprehensive assessment of functional and radiological lung evaluation.
Acute Phase
In our series, patients who received a higher respiratory support during acute phase had a higher prevalence of cardiovascular comorbidities, a lower PaO2/FiO2 nadir, a higher HRCT extension score, and a higher lactate dehydrogenase at admission (p < 0.001) (Table 1). We also found that more patients of the high ventilatory support group received therapies (hydroxychloroquine, azithromycin, steroids, antivirals, and tocilizumab) compared to the low ventilatory support group, probably due to the severity of pneumonia. Anyway, these results may be misunderstood as treatment was not standardized (patient received different dosages, different duration, and at different times of the disease).
Symptoms
From a clinical point of view, our study reports that 64 and 42% of patients were still symptomatic at 2 and 6 months, respectively. At 2 months Carfì et al. [1] reported that 87% of patients complained persistence of at least one symptom; at 4 months; 51% of patients declared at least one symptom in a French series [8]; 69% of patients complained at least 1 symptom at 5 months in another Italian study [9], whilst 76% of patients reported at least 1 symptom at 6 months in a large Chinese series [7]. The percentage of symptomatic patients in our study is lower than in the others; this is probably due to the absence of psychological or cognitive items in our checklist (e.g., sleep disturbances, anxiety or depression, memory difficulties, and concentration problems). In our series, exertional dyspnoea (44%), fatigue (13%), and cough (10%) were the most prevalent symptoms, and this is consistent with other studies [1, 8, 9].
Pulmonary Function Tests
Our series shows a progressive improvement of spirometry from 2 to 6 months after discharge. Li et al. [12] showed a similar improvement soon after discharge (2 and 4 weeks); therefore, this initial functional gain seems to last, even in the absence of any therapy, for several months.
Impairment of diffusion capacity represents the most common abnormality of lung function in discharged COVID-19 survivors (51% of patients) according to Mo et al. [2], with increasing impairment as the acute pulmonary involvement increased in severity. Our data show that at 6-month DLCO is reduced in 57% of patients and that DLCO was significantly worse in patients with lower PaO2/FiO2 nadir during hospitalization (p < 0.05, Table 3). These results are consistent with Huang et al. [7], Morin et al. [8] and an Italian series [10]. Therefore, DLCO impairment seems to be the functional hallmark of COVID-19 and it is worse the worst acute pneumonia was, whatever is the way to categorize the severity of acute pneumonia (PaO2/FiO2 or highest ventilatory support).
Six-Minute Walking Test . Our results show a significant improvement of 6MWD from 2 (418 m) to 6 months (489 m, p < 0.001). 6MWD at 6 months is consistent with the distances reported by 2 other Italian series (Anastasio et al. [9] 500–520 m and Faverio et al. [10] 450–485 m). Another Italian study proved the efficacy of formal rehabilitation in increasing 6MWD in patients with severe COVID-19 pneumonia soon after discharge from the intensive care unit [4]. Six months after the acute phase, Liu et al. [13] performed a randomized-controlled trial (6-week respiratory rehabilitation training vs. no rehabilitation) and showed a statistically significant improvement of 6MWD only in the intervention group. These data suggest that 6MWD improves even out of any formal rehabilitation program within 6 months from discharge, but as rehabilitation has proved its efficacy, this window of time might be an opportunity for active training.
Correlating exercise performance during follow-up and severity of the acute phase, our results show that patients with a more severe acute pneumonia (PaO2/FiO2 nadir ≤200) has a significantly lower 6MWD and 6MWD % predicted at 2 but not at 6 months (Table 4). This result is consistent with an Italian series that found no difference in 6MWD at 6 months between groups divided according to the highest support during hospitalization [10].
Although distance is the most measured variable during 6MWT as it represents the most reliable measure of exercise capacity and has an important prognostic value, we also reported other variables related to 6MWT, such as lowest SpO2, desaturation % and DSP [17, 19]. Our results show that patient with a more severe acute pneumonia (PaO2/FiO2 nadir ≤200) has a lower minimum SpO2 and a higher desaturation % during 6MWT at both 2 and 6 months (Table 4). This result is consistent with Anastasio et al. [9] who found that lowest SpO2 during 6MWT at 6 months is still worse in patients with more severe acute pneumonia. Taken together, these data suggest that at 6 months the patients with more severe acute pneumonia seem to have annulled the 6MWD gap with those with milder acute disease while gas exchange is still impaired. Causes of gas exchange impairment might be persistent interstitial pathology or ventilation/perfusion (V/Q) mismatch caused by chronic thromboembolism. At 6 months, we found an inverse correlation between the extent of HRCT involvement and 6MWT parameters (6MWD, lowest SpO2, and DSP) (Table 5), suggesting that a worse exercise performance might be linked to more severe parenchymal abnormalities. The same table shows the correlation between HRCT extension score and PFTs, demonstrating a significant inverse correlation with TLC and DLCO (p < 0.05); a better correlation was found with TLC. Taken together the significant relation between HRCT extension score, PFTs (above all TLC), and 6MWT may suggest a prevalent role of postinfectious ILD in the impairment of exercise performance, whilst long-term vascular involvement may play a minor role. This hypothesis is quite speculative, but the absence of significant long-term cardiovascular alterations has been also suggested in 2 larger post-COVID series [7, 8]. Obviously, other studies are needed to confirm this assumption.
Imaging
Chest-x-Ray. Since CT scans were overloaded during the first wave of COVID-19, even if CXR has limited sensitivity and specificity for COVID-19, several patients were monitored with CXR to assess acute complications (e.g., pneumothorax) [27]. An Italian group validated a semi-quantitative assessment of pulmonary involvement of CXR in hospitalized patients with COVID-19, called Brixia Score [21, 22]. The median Brixia CXR score reported in literature during acute phase is 7 [21], while our data report a mean 2-month Brixia score of 3 (±3), suggesting an improvement of radiological abnormalities. In our series, we include CXR at 2-month evaluation to explore the possibility of medium-term complication (e.g., lung abscesses and pleural effusions) and to assess the presence of parenchymal opacities, interstitial thickening, or fibrotic lines.
Lung Ultrasound. Even if the role of LUS in the acute phase of COVID-19 is globally accepted, the utility of LUS in long-term follow-up of COVID-19 has not yet been defined. Our results show that at 2 months from acute phase 59% of patients have an interstitial pulmonary syndrome. These results are consistent with an Italian study which found interstitial pulmonary syndrome in 63% of 38 patients at 3 months after the acute phase of COVID-19 [28].
High-Resolution Computed Tomography. The radiological evolution of COVID-19 pneumonia starts from a prevalence of GGO and reticulation with a predominantly peripheral, middle-lower, and posterior distribution; then it goes through an increase of GGO plus reticular pattern and consolidation pattern with the appearance of subpleural lines and bronchus distortion; after about 14 days from the onset of symptoms the lung involvement starts to decrease [5]. One month after discharge, the abnormalities in the lungs (GGO, consolidation, interlobular septal thickening, and irregular lines) seem to gradually reduce [6], but data at 4 months reveal the persistence of abnormalities in most patients (58% of non-intubated and in 75% of intubated patients) [8]. Our results show that lung abnormalities at 6 months decrease, with a significant reduction in the severity score from 13 (acute phase) to 6 (at 6 months) (p < 0.001). Furthermore, a higher score at 6 months was found in patients with PaO2/FIO2 nadir <200 (p < 0.001). These data at 6 months are consistent with the results of a large Chinese series that show a trend to larger extension of HRCT impairment in patients with more severe acute pneumonia [7]. At 6 months, the most common HRCT patterns reported by Huang et al. [7] were GGO (≥40%) and irregular lines (≥10%). Our series shows similar results, with GGO in 57% and lines in 18% of patients.
Limitations
Our investigation has some limitations. Firstly, it is an uncontrolled study, including only COVID-19 patients without a non-COVID-19 comparison group. Then we have no PFTs and HRCT before the disease and at discharge, making any firm conclusion about the fulfilment and the time of recovery quite speculative. Very few patients in this series had chronic respiratory symptoms or pre-existing lung diseases (ILD and COPD) before COVID-19, so it is impossible to have any baseline lung function test or HRCT. In addition, pre-existing lung diseases may affect the ability to distinguish the extent of post-COVID alterations from functional impairment. During the first wave of COVID-19 in Italy, there was a shortage of beds, which induced precocious discharges from hospitals; furthermore, the staff was almost completely dedicated to the care of acute hospitalized patients and the guidelines for a safe performance of lung function tests were not yet available so lung function studies before discharge were not a priority at that time. The same reason justifies the absence of DLCO at 2 months. In addition, unluckily, some radiological data are missing (I.e. not every patient underwent all the imaging) but this was a “real life” study, begun when the first pandemic wave was not yet over, so it was not always possible to match the patients' requirements with the machines' availability. The lack of LUS and CXR in certain patients makes impossible to perform a full-correlation analysis on these tests. Finally, we do not include therapies administered during hospitalization in the follow-up correlation analysis because our population did not receive homogeneous treatment (e.g., type of steroid used, number of treatment days, and type of tapering), and therefore, any speculation regarding the influence of the treatment on the recovery trajectory would be biased.
Conclusion
This study suggests that COVID-19 patients improve between 2 and 6 months after discharge. A mild defect in gas exchange and of exercise capacity seems to be the functional hallmark of aftermath. According to the results of our study, severity of acute pneumonia is related to the severity of functional and radiological alterations (Tables 3, 4). This correlation is consistent with 2 long-term follow-up studies [7, 8] and endorses BTS guidance to pay more attention at follow-up of patients with more severe pneumonia [14, 15].
Statement of Ethics
The research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. The study was approved by the local Ethical Committee (CE-AVEC, Comitato Etico Area Vasta Emilia Centro) (n. 714/2020). Written informed consent was obtained from patients to participate in the study.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
No funding was received.
Author Contributions
M.F., L.F., and I.P. participated to the patients' enrolment, study design and writing, and outpatient visit. S.N. participated in study design and writing. S.B., S.B., and F.D. participated to the patients' enrolment and outpatient visit, V.S., C.M., and M.R.R. performed radiological studies; C.M. and C.S. performed LUS studies. Collaborators: Alice Rossi (Alma Mater Studiorum - Università di Bologna).
Data Availability Statement
All data generated or analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.
References
- 1.Carfì A, Bernabei R, Landi F, Gemelli Against COVID-19 Post-Acute Care Study Group Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324((6)):603–5. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mo X, Jian W, Su Z, Chen M, Peng H, Peng P, et al. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J. 2020;55:2001217. doi: 10.1183/13993003.01217-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nusair S. Abnormal carbon monoxide diffusion capacity in COVID-19 patients at time of hospital discharge. Eur Respir J. 2020;56:2001832. doi: 10.1183/13993003.01832-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zampogna E, Paneroni M, Belli S, Aliani M, Gandolfo A, Visca D, et al. Pulmonary rehabilitation in patients recovering from COVID-19. Respiration. 2021;100((5)):416–22. doi: 10.1159/000514387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou S, Zhu T, Wang Y, Xia L. Imaging features and evolution on CT in 100 COVID-19 pneumonia patients in Wuhan, China. Eur Radiol. 2020;30((10)):5446–54. doi: 10.1007/s00330-020-06879-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu C, Ye L, Xia R, Zheng X, Yuan C, Wang Z, et al. Chest computed tomography and clinical follow-up of discharged patients with COVID-19 in Wenzhou City, Zhejiang, China. Ann Am Thorac Soc. 2020 Oct;17((10)):1231–7. doi: 10.1513/AnnalsATS.202004-324OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6 months consequences of COVID19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–32. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Writing Committee for the COMEBAC Study Group. Morin L, Savale L, Pham T, Colle R, Figueiredo S, et al. Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA. 2021;325((15)):1525–34. doi: 10.1001/jama.2021.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anastasio F, Barbuto S, Scarnecchia E, Cosma P, Fugagnoli A, Rossi G, et al. Medium-term impact of COVID-19 on pulmonary function, functional capacity and quality of life. Eur Respir J. 2021 Feb 11;58((3)):2004015. doi: 10.1183/13993003.04015-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faverio P, Luppi F, Rebora P, Busnelli S, Stainer A, Catalano M, et al. Six-month pulmonary impairment after severe COVID-19: a prospective, multicentre follow-up study. Respiration. 2021 Aug 19;100((11)):1078–87. doi: 10.1159/000518141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myall KJ, Mukherjee B, Castanheira AM, Lam JL, Benedetti G, Mak SM, et al. Persistent post-COVID-19 inflammatory interstitial lung disease: an observational study of corticosteroid treatment. Ann Am Thorac Soc. 2021;18((5)):799–806. doi: 10.1513/AnnalsATS.202008-1002OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Wang C, Kou S, Luo P, Zhao M, Yu K. Lung ventilation function characteristics of survivors from severe COVID-19: a prospective study. Crit Care. 2020 Jun 6;24((1)):300. doi: 10.1186/s13054-020-02992-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu K, Zhang W, Yang Y, Zhang J, Li Y, Chen Y. Respiratory rehabilitation in elderly patients with COVID-19: a randomized controlled study. Complement Ther Clin Pract. 2020 May;39:101166. doi: 10.1016/j.ctcp.2020.101166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.BTS . BTS guidance on respiratory follow up of patients with a clinico-radiological diagnosis of COVID-19 pneumonia [Internet] [updated 2020 May 5]. Available from: https://brit-thoracic.org.uk/about-us/covid-19-information-for-the-respiratory-community/ Accessed 2021 Apr 7. [Google Scholar]
- 15.George PM, Barratt SL, Condliffe R, Desai SR, Devaraj A, Forrest I, et al. Respiratory follow-up of patients with COVID-19 pneumonia. Thorax. 2020 Nov;75((11)):1009–16. doi: 10.1136/thoraxjnl-2020-215314. [DOI] [PubMed] [Google Scholar]
- 16.Lessmann N, Sánchez CI, Beenen L, Boulogne LH, Brink M, Calli E, et al. Automated assessment of COVID-19 reporting and data system and chest CT severity scores in patients suspected of having COVID-19 using artificial intelligence. Radiology. 2021 Jan;298((1)):E18–28. doi: 10.1148/radiol.2020202439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holland AE, Spruit MA, Troosters T, Puhan MA, Pepin V, Saey D, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014 Dec;44((6)):1428–46. doi: 10.1183/09031936.00150314. [DOI] [PubMed] [Google Scholar]
- 18.Gibbons WJ, Fruchter N, Sloan S, Levy RD. Reference values for a multiple repetition 6-minute walk test in healthy adults older than 20 years. J Cardiopulm Rehabil. 2001 Mar–Apr;21((2)):87–93. doi: 10.1097/00008483-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Singh SJ, Puhan MA, Andrianopoulos V, Hernandes NA, Mitchell KE, Hill CJ, et al. An official systematic review of the European Respiratory Society/American Thoracic Society: measurement properties of field walking tests in chronic respiratory disease. Eur Respir J. 2014 Dec;44((6)):1447–78. doi: 10.1183/09031936.00150414. [DOI] [PubMed] [Google Scholar]
- 20.Johnson JD, Theurer WM. A stepwise approach to the interpretation of pulmonary function tests. Am Fam Physician. 2014 Mar 1;89((5)):359–66. [PubMed] [Google Scholar]
- 21.Borghesi A, Maroldi R. COVID-19 outbreak in Italy: experimental chest X-ray scoring system for quantifying and monitoring disease progression. Radiol Med. 2020 May;125((5)):509–13. doi: 10.1007/s11547-020-01200-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borghesi A, Zigliani A, Masciullo R, Golemi S, Maculotti P, Farina D, et al. Radiographic severity index in COVID-19 pneumonia: relationship to age and sex in 783 Italian patients. Radiol Med. 2020 May;125((5)):461–4. doi: 10.1007/s11547-020-01202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Via G, Storti E, Gulati G, Neri L, Mojoli F, Braschi A. Lung ultrasound in the ICU: from diagnostic instrument to respiratory monitoring tool. Minerva Anestesiol. 2012 Nov;78((11)):1282–96. [PubMed] [Google Scholar]
- 24.Zanforlin A, Strapazzon G, Falk M, Gallina V, Viteritti A, Valzolgher L, et al. Lung ultrasound in the emergency department for early identification of COVID-19 pneumonia. Respiration. 2021;100((2)):145–53. doi: 10.1159/000512782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sahu AK, Mathew R, Bhoi S, Sinha TP, Nayer J, Aggarwal P. Lung sonographic findings in COVID-19 patients. Am J Emerg Med. 2021 Jul;45:324–8. doi: 10.1016/j.ajem.2020.08.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, et al. International Liaison Committee on Lung Ultrasound (ILC-LUS) for International Consensus Conference on Lung Ultrasound (ICC-LUS). International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012 Apr;38((4)):577–91. doi: 10.1007/s00134-012-2513-4. [DOI] [PubMed] [Google Scholar]
- 27.Larici AR, Cicchetti G, Marano R, Merlino B, Elia L, Calandriello L, et al. Multimodality imaging of COVID-19 pneumonia: from diagnosis to follow-up. A comprehensive review. Eur J Radiol. 2020;131:109217. doi: 10.1016/j.ejrad.2020.109217. Erratum in: Eur J Radiol. 2021 Jan;134:109364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giovannetti G, De Michele L, De Ceglie M, Pierucci P, Mirabile A, Vita M, et al. Lung ultrasonography for long-term follow-up of COVID-19 survivors compared to chest CT scan. Respir Med. 2021 May;181:106384. doi: 10.1016/j.rmed.2021.106384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.