Significance Statement
This prespecified analysis of the SONAR trial in patients with type 2 diabetes and CKD demonstrated the early albuminuria reduction during an open-label, 6-week run-in period with atrasentan was associated with a reduced risk for long-term kidney outcomes in patients who continued atrasentan after randomization. But because the early albuminuria reduction also associated with long-term kidney outcomes in patients who transitioned from atrasentan to placebo at randomization, atrasentan’s effect on the primary kidney outcome was consistent, regardless of the early albuminuria change, suggesting the early albuminuria response is not a causal predictor for atrasentan’s nephroprotective effect. However, the variable UACR trajectory in the placebo arm, aspects of the SONAR trial design, day-to-day variability in albuminuria, and potential long-lasting effects of atrasentan may have contributed.
Keywords: clinical trial, albuminuria, Endothelin Receptor Antagonist, type 2 diabetes, SONAR
Abstract
Background
Whether early reduction in albuminuria with atrasentan treatment predicts its long-term kidney-protective effect is unknown.
Methods
To assess the long-term effects on kidney outcomes of atrasentan versus placebo in the SONAR trial, we enrolled patients who had type 2 diabetes and CKD (stage 2–4) and a urinary albumin creatinine ratio (UACR) of 300–5000 mg/g; participants were receiving maximum tolerated renin-angiotensin system inhibition. After 6 weeks exposure to 0.75 mg/day atrasentan (enrichment period), participants were randomized (stratified by UACR response during enrichment, ranging from ≤60% to >0%) to continue atrasentan or transition to placebo. Primary kidney outcome was a composite of sustained serum creatinine doubling or ESKD.
Results
UACR response to atrasentan during enrichment persisted throughout the double-blind treatment phase and predicted the primary kidney outcome, whereas UACR levels with placebo remained below pre-enrichment values in the two highest UACR response strata, and exceeded pre-enrichment values in the two lowest strata. As a result, early UACR response to atrasentan during enrichment was also associated with the primary kidney outcome during placebo. Accordingly, the predictive effect of early albuminuria changes during atrasentan was eliminated after placebo correction, leading to a consistent relative risk reduction for the primary kidney outcome with atrasentan compared with placebo, irrespective of the initial UACR response. The difference between atrasentan and placebo in UACR during double-blind treatment was also consistent across UACR response strata.
Conclusions
Our findings do not support UACR response as a causal predictor of atrasentan’s treatment effect. However, the variable trajectory in UACR with placebo, aspects of the trial design, day-to-day variability in albuminuria, and potential long-lasting effects of atrasentan may have contributed.
CKD is recognized as a growing public health problem.1,2 Clinical practice guidelines recommend early detection for CKD and appropriate treatment to slow progression of kidney function decline and reduce the risk of long-term complications.3 Albuminuria is a strong risk marker of CKD progression and cardiovascular complications. Screening and monitoring of albuminuria is therefore an important component in the management of patients with CKD.
Various drugs such as angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) and the more recent sodium glucose cotransporter 2 inhibitors and mineralocorticoid receptor antagonists reduce albuminuria and the risk of kidney failure and cardiovascular events.4,5 Observational analyses from clinical trials demonstrating the efficacy of these interventions have consistently shown the early reduction in albuminuria, achieved within the first months of treatment, is associated with long-term kidney protection.6–9 On the basis of these studies, it has been recommended to monitor albuminuria after initiation of these drugs to guide clinical management.10 However, these observational analyses from clinical trials are prone to selection bias and residual confounding. It is therefore possible that the lower risk of kidney failure among patients in whom albuminuria was reduced is caused by factors unrelated to the reduction in albuminuria. To overcome this bias, one should ideally design a clinical trial that first records albuminuria responses during exposure in an active run-in period, subsequently randomly assigns patients to continue the drug or transition to a placebo treatment, and analyzes the long-term drug effect relative to placebo in subgroups, according to the response in albuminuria during the run-in period.
The selective endothelin A receptor antagonist atrasentan has been demonstrated to reduce albuminuria in patients with type 2 diabetes and CKD.11 Of note, the albuminuria-lowering effect varied considerably between patients.11 This favorable effect of atrasentan on albuminuria led to the conduct of a confirmatory clinical trial, the Study of Diabetic Nephropathy with Atrasentan (SONAR) trial, to assess the long-term efficacy and safety of atrasentan. The primary results were reported previously and demonstrated that atrasentan significantly reduced the risk of kidney failure compared with placebo in patients with type 2 diabetes and CKD.12 The design of the SONAR trial included an open-label active run-in period (termed a response enrichment period), during which all patients received atrasentan 0.75 mg once daily for 6 weeks. After the response-enrichment period, eligible patients were randomly assigned to placebo or atrasentan, stratified by the degree of albuminuria lowering during enrichment. This strategy offered the opportunity to investigate in a placebo-controlled design whether the early reduction in albuminuria to atrasentan predicts its long-term kidney protective effect. We therefore undertook a prespecified analysis of the effect of atrasentan according to the degree of albuminuria response achieved during the response-enrichment period of the SONAR trial.
Methods
Study Design and Participants
The SONAR trial was a multicentre, double-blind, placebo-controlled, randomized trial evaluating the effects of atrasentan on kidney outcomes in subjects who had type 2 diabetes and CKD. The SONAR trial is registered with ClinicalTrials.gov, number NCT01858532, and the trial protocol including a detailed description of the trial design and statistical analysis plans has been published previously, along with the primary SONAR trial article.12,13 In brief, patients with type 2 diabetes mellitus and CKD, defined as eGFR of 25–75 ml/min per 1.73m2 and urinary albumin-creatinine ratio (UACR) of 300–5000 mg/g, who were receiving a maximum tolerated dose of an ACE-inhibitor or ARB, were eligible. Patients prone to fluid retention, defined as B-type natriuretic peptide (BNP) > 200 pg/ml, prior hospital admission for heart failure, or a history of severe edema, could not participate in the trial. After screening, eligible patients received 0.75 mg atrasentan once daily during the 6 weeks response enrichment period, aimed to select patients that were likely to respond to atrasentan, defined as an UACR reduction of ≥30%, and to exclude patients that were prone to atrasentan-induced fluid retention, defined as an increase of ≥3 kg in body weight or an increase in BNP to ≥300 pg/ml.
All responder patients who tolerated atrasentan and a selection of patients who were nonresponders subsequently proceeded to the randomization visit and were assigned in a 1:1 ratio to continue atrasentan 0.75 mg/day or to transition to placebo. Randomization was performed centrally through an interactive voice response system on the basis of a computer-generated randomization schedule. A stratified randomization scheme was used to ensure balance in treatment allocation within geographic regions and with baseline UACR levels (≤ or >1000 mg/g). Additionally, randomization was stratified by UACR change during the response-enrichment period: ≤-60%; -60% to ≤-45%; -45% to ≤-30% (classified as UACR responders); and -30% to ≤-15%; -15% to ≤0%; >0% (classified as UACR nonresponders). This stratification by different UACR response levels was prespecified to assess if the effect of atrasentan compared with placebo on the primary kidney outcome depended on the UACR response during response enrichment.
The median total follow-up period was 2.2 years until the last trial visits (either in clinic or telephone), which occurred by March 29, 2018. Local institutional ethics committees approved the trial protocols at each site. All participants provided written informed consent. The trial was conducted according to the principles outlined in the Declaration of Helsinki.
Outcomes
The primary outcome used to evaluate the efficacy of atrasentan in delaying the progression of CKD was the time to the first occurrence of any of the following components of a composite end point consisting of: doubling of serum creatinine (confirmed by a second serum creatinine measurement ≥30 days later), onset of ESKD (defined as chronic dialysis for >90 days, kidney transplantation, or eGFR <15 ml/min per 1.73m2, confirmed by a second measurement ≥90 days later), or death from kidney failure. An independent end point committee adjudicated all potential outcomes using rigorous end-point definitions.
Statistical Analysis
The analytical approach and power calculation have been published.12 The trial was originally powered to capture 425 primary outcomes that provided 90% power to detect a hazard ratio (HR) of 0.73. As described previously, the sponsor decided to stop the trial prematurely due to a lower than expected event rate. At completion of the trial, 184 primary renal events had occurred, providing >80% power to detect an HR of 0.66.12
For this prespecified analysis, we performed Cox proportional hazard regression to estimate the HR and the 95% confidence interval (95% CI) for atrasentan, compared with placebo for the primary kidney outcomes in each UACR response stratum. For patients who experienced more than one event during follow-up, survival time to the first relevant end point was used. The treatment effect in the model was adjusted for log-transformed UACR values, serum albumin, age, and eGFR at randomization. To assess whether the treatment effect of atrasentan compared with placebo during double-blind treatment varied by different UACR response levels, we performed prespecified tests for heterogeneity by adding interaction terms between the UACR response stratum, as a categorical variable, and randomized treatment assignment to the relevant Cox models. We performed post-hoc Cox proportional hazard regression to assess the associations between changes in UACR during the response-enrichment period and the primary kidney outcome within the atrasentan and placebo treatment group separately. The model was adjusted for age, sex, race, baseline systolic and diastolic BP, body weight, HbA1c, eGFR, albumin, hemoglobin log-transformed UACR, cardiovascular disease history, diuretic and beta-blocker use at baseline, and change in systolic BP during the response-enrichment period. We verified Cox proportional hazard assumptions by visual inspection of the log(-log[survival]) curve and by adding a time by treatment interaction to the Cox model. These analyses showed no violations of the model assumptions.
We calculated the difference of change in the geometric mean UACR between the atrasentan and placebo groups during double-blind treatment with a repeated mixed-effects models to determine the effect of atrasentan versus placebo on UACR. We determined the effect of atrasentan relative to placebo on UACR in the overall population and in each UACR response stratum. The mixed-effects model included treatment allocation and time as factor, and an interaction term between treatment allocation and time. The model also included UACR response stratum as factor and interaction terms between treatment assignment and UACR response stratum, time and UACR response stratum, and time, treatment assignment, and UACR response stratum. The model was adjusted for the log-transformed baseline UACR level and interaction term between time and baseline log-transformed UACR. The variance-covariance matrix was assumed to be unstructured, that is, purely data dependent. The same model was used to calculate systolic BP differences between atrasentan and placebo during double-blind treatment.
All analyses were conducted using SAS, version 9.4. A two-sided P value <0.05 was considered statistically significant and no adjustment was made for multiplicity.
Results
Baseline Characteristics
The SONAR trial randomized 3668 participants, of whom 2648 were UACR responders (≥30% UACR reduction) and 1020 were nonresponders (<30% UACR reduction). At baseline the median UACR was 829 mg/g (25th to 75th percentile 457–1556). A total of 2055 (56.0%) participants had a UACR <1000 mg/g and 1613 (44.0%) a UACR ≥1000 mg/g. Nearly all participants (98.5%) were using an inhibitor of the renin-angiotensin-aldosterone system (RAAS) at baseline. Across progressively larger UACR response strata, participants were older, had a higher eGFR, serum albumin, and a lower body weight. Median UACR was progressively lower in higher UACR response strata (Table 1). eGFR values before screening were recorded in a random selection of 532 patients whose characteristics were similar to all randomized participants (Supplemental Table 1). The pretrial eGFR decline in these participants was similar between UACR responders with ≥30% UACR reduction during response enrichment compared with UACR nonresponders, with <30% UACR reduction during response enrichment (4.7 ml/min per 1.73m2 per year, SD 10.3, versus 5.5 ml/min per 1.73m2, SD 8.7; P=0.39). Baseline characteristics were balanced between atrasentan and placebo groups within each enrichment UACR response stratum (Supplemental Table 2).
Table 1.
Patient characteristics at start of the enrichment period according to predefined UACR response strata
| Characteristics | UACR ≥60% | UACR 45%–60% | UACR 30%–45% | UACR 15–30% | UACR 0%–15% | UACR <0% | P for trend |
|---|---|---|---|---|---|---|---|
| N patients (%) | 591 | 968 | 1089 | 479 | 286 | 255 | |
| Age, yrs | 65.6 (9) | 64.6 (9) | 64.6 (9) | 64.5 (9) | 63.1 (9) | 62.9 (9) | <0.001 |
| Sex | 0.215 | ||||||
| Male | 420 (71.1) | 716 (74.0) | 829 (76.1) | 356 (74.3) | 219 (76.6) | 182 (71.4) | |
| Female | 171 (28.9) | 252 (26.0) | 260 (23.9) | 123 (25.7) | 67 (23.4) | 73 (28.6) | |
| Body weight, kg | 84.3 (19) | 84.2 (20) | 85.1 (19) | 87.4 (20) | 85.9 (19) | 87.2 (21) | 0.028 |
| Systolic BP, mmHg | 137.4 (15) | 136.4 (15) | 135.7 (15) | 135.5 (15) | 135.5 (15) | 137.2 (16) | 0.185 |
| Diastolic BP, mmHg | 75.0 (10) | 74.8 (10) | 75.0 (10) | 74.3 (10) | 75.1 (10) | 75.8 (9) | 0.482 |
| Hba1c (%) | 7.6 (1.5) | 7.5 (1.4) | 7.6 (1.4) | 7.6 (1.5) | 7.7 (1.6) | 7.6 (1.5) | 0.543 |
| Serum creatinine, µmol/L | 141 (42) | 147 (42) | 152 (42) | 158 (48) | 155 (45) | 152 (42) | <0.001 |
| eGFR, ml/min per 1.73m2 | 45.6 (15) | 44.2 (14) | 42.6 (13) | 41.2 (15) | 42.4 (14) | 42.3 (12) | <0.001 |
| Serum albumin, g/L | 39.9 (3.4) | 39.3 (3.5) | 39.1 (3.5) | 38.7 (3.8) | 38.3 (3.9) | 38.7 (3.7) | <0.001 |
| Hemoglobin, g/L | 129 (17) | 129 (17) | 130 (17) | 130(17) | 128 (18) | 128 (18) | 0.103 |
| BNP, pg/mL | 56 (27–95) | 49 (26–89) | 46 (25–82) | 46 (25–80) | 45 (25–85) | 47 (27–85) | 0.099 |
| UACR, mg/g | 660 (389–1188) | 806 (448–1431) | 884 (496–1619) | 955 (486–1879) | 1070 (545–2176) | 800 (406–1475) | <0.001 |
| Medication usea | |||||||
| Diuretics | 500 (84.6) | 817 (84.4) | 903 (82.9) | 396 (82.7) | 234 (81.8) | 215 (84.3) | 0.806 |
| Beta-blockers | 271 (45.9) | 419 (43.3) | 428 (39.3) | 189 (39.5) | 121 (42.3) | 103 (40.4) | 0.110 |
| Lipid-lowering drugs | 453 (76.6) | 761 (78.6) | 879 (80.7) | 399 (83.3) | 221 (77.3) | 200 (78.4) | 0.086 |
Numeric variables are presented as mean (SD) or median (25th to 75th percentile) where appropriate. Categorical variables are presented as counts.
All patients had to be on a maximum tolerated dose of an ACE-inhibitor or ARB. P for trend is test for trend across the UACR response strata.
Greater reductions in BP and eGFR during enrichment were observed in higher UACR response strata, whereas changes in body weight and BNP were similar across UACR response strata (Supplemental Table 3). A total of 3104 participants completed the trial, of whom 2275 were UACR responders with a medium follow-up of 2.2 years, and 829 were UACR nonresponders with a median follow-up of 2.3 years.
Early UACR Change and Kidney Outcomes
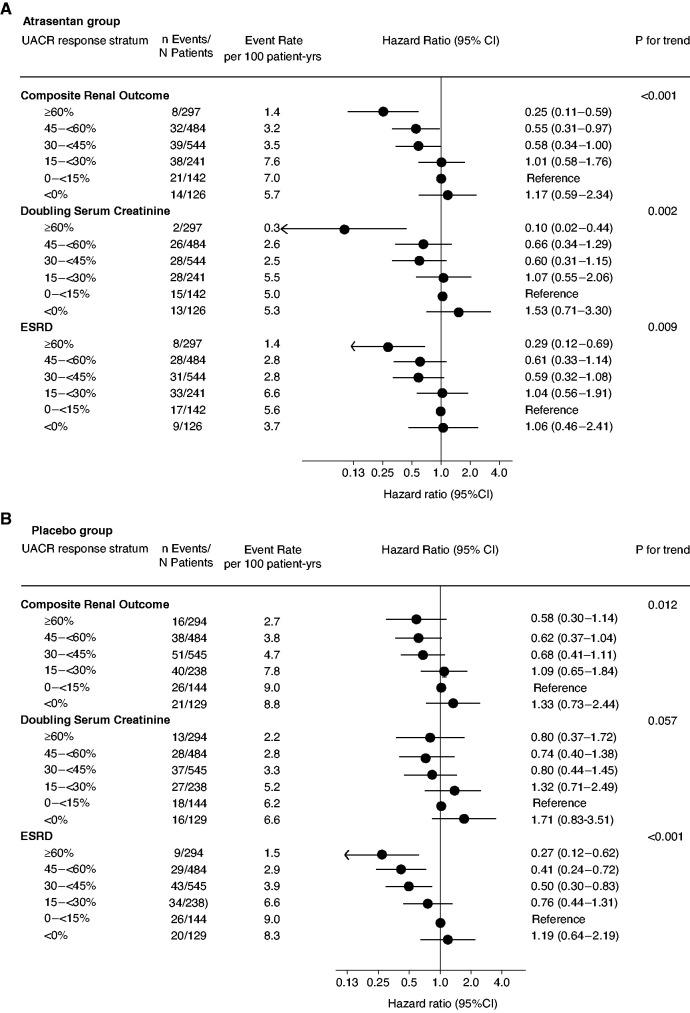
To assess whether changes in UACR during atrasentan were associated with subsequent kidney outcomes, we first analyzed the atrasentan group. Among participants who were randomized to continue atrasentan, we observed that the change in UACR during the response enrichment period persisted during the double-blind treatment phase across all UACR response strata. For example, the geometric mean percentage UACR reduction among participants in the ≥60% UACR response stratum was -70.5% (95% CI, -71.6 to -69.4) during response enrichment and -61.4% (95% CI, -66.1 to -56.0) during the double-blind treatment phase (Table 2). In these participants, the incidence of the primary kidney outcome was lower in higher UACR response strata (Figure 1A). In a multivariable Cox model adjusting for differences in patient characteristics across UACR response strata, participants with a >60% decrease in UACR during enrichment had an multivariable adjusted 75% lower hazard for the composite kidney outcome (HR, 0.25; 95% CI, 0.11 to 0.59) compared with participants with a UACR increase <15% (Figure 1A).
Table 2.
Percent change (95%CI) in UACR in the atrasentan and placebo groups during response enrichment, double-blind treatment, and after discontinuation of atrasentan
| UACR Response Strata | Atrasentan | Placebo | ||||
|---|---|---|---|---|---|---|
| Δ UACR from Pre-enrichment to End of Enrichment | Δ UACR from Pre-enrichment to Double-blind Treatment | Δ UACR from Last On-treatment to Off-treatment | Δ UACR from Pre-enrichment to End of Enrichment | Δ UACR from Pre-enrichment to Double-blind Treatmenta | Δ UACR from Last On-treatment to Off-treatment | |
| ≥60% reduction | −70.5 (-71.6, -69.4) | −61.4 (-66.1, -56.0) | 49.0 (43.6, 53.9) | −71.6 (-72.7, -70.6) | −28.1 (-36.8, -18.3) | 53.2 (49.7, 56.5) |
| 45–60% reduction | −52.9 (-54.3, -51.5) | −44.8 (-50.0, -39.1) | 48.1 (43.8, 52.0) | −52.2 (-53.6, -50.8) | −15.6 (-23.5, -7.0) | 38.8 (34.1, 41.2) |
| 30–45% reduction | −38.6 (-40.3, -36.9) | −36.6 (-42.4, -30.2) | 42.0 (37.4, 46.2) | −39.1 (-40.8, -37.4) | 10.6 (0.8, 21.4) | 35.4 (31.8, 38.8) |
| 15–30% reduction | −23.9 (-26.9, -20.7) | −30.7 (-39.5, -20.7) | 38.2 (30.5, 45.0) | −23.9 (-27.0, -20.7) | 26.7 (11.2, 44.4) | 26.2 (19.9, 31.9) |
| 0–15% reduction | −6.5 (-11.4, -1.3) | −0.2 (-16.5, 19.3) | 36.5 (26.1, 45.4) | −11.4 (-16.0, -6.5) | 40.8 (17.5, 68.8) | 29.9 (22.0, 36.9) |
| ≥0% increase | 23.7 (16.9, 31.0) | 18.4 (-4.1, 46.1) | 36.0 (24.1, 46.1) | 36.3 (28.8, 44.2) | 48.3 (20.8, 81.9) | −0.9 (-13.1, 9.9) |
We had expected UACR levels in the placebo group would return to baseline pre-enrichment values and the change in UACR from pre-enrichment to double-blind treatment would be 0%. However, UACR levels stayed low in the responder strata (indicated by the negative signs) and increased to values above pre-enrichment in the nonresponder strata.
Figure 1.
Association between changes in UACR during the 6-weeks response-enrichment period and subsequent kidney outcomes recorded during the double-blind treatment period. (A) The association in participants who continued atrasentan during the double-blind treatment period. (B) The association in participants who transitioned from atrasentan to placebo at randomization.
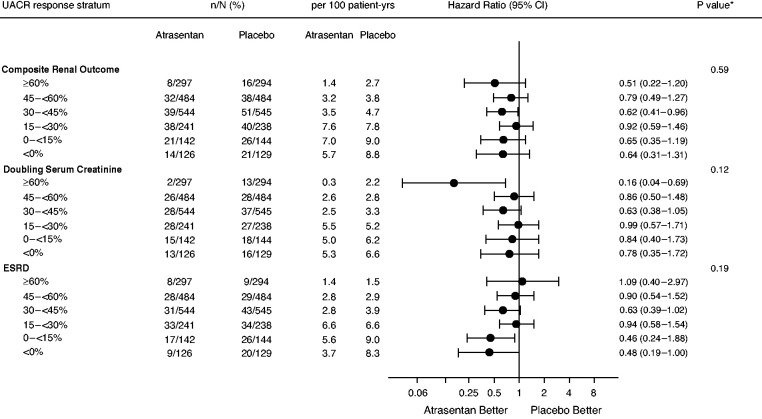
To assess whether the association between UACR responses to atrasentan and the primary kidney outcome persisted after taking into account the placebo group, we analyzed the effect of atrasentan compared with placebo in each UACR response stratum. The primary kidney outcome occurred in 152 (8.3%) participants in the atrasentan group and 192 (10.5%) participants in the placebo group (HR, 0.72; 95% CI, 0.58 to 0.89). The relative effect of atrasentan compared with placebo on the primary kidney outcome was consistent across UACR enrichment response strata (P for heterogeneity 0.59; Figure 2). The same was true for the components of the primary kidney outcome: sustained doubling of serum creatinine (Figure 2, P for heterogeneity 0.12) and ESKD (Figure 2, P for heterogeneity 0.19). No heterogeneity was observed for any of these outcomes when interaction tests were undertaken using log-transformed UACR as a continuous variable. Atrasentan reduced the mean rate of change in eGFR compared with placebo by 0.65 ml/min per 1.73m2 per year (95% CI, 0.28 to 1.01; P<0.001). This effect was consistent across UACR enrichment response strata (P for heterogeneity 0.91; Supplemental Figure 1). The effect of atrasentan compared with placebo on heart failure hospitalizations was also consistent across UACR response strata (P for heterogeneity 0.72; Supplemental Figure 2).
Figure 2.
Effects of atrasentan compared with placebo on the primary kidney end point and its components during the double-blind treatment period according to UACR response during the response enrichment period.
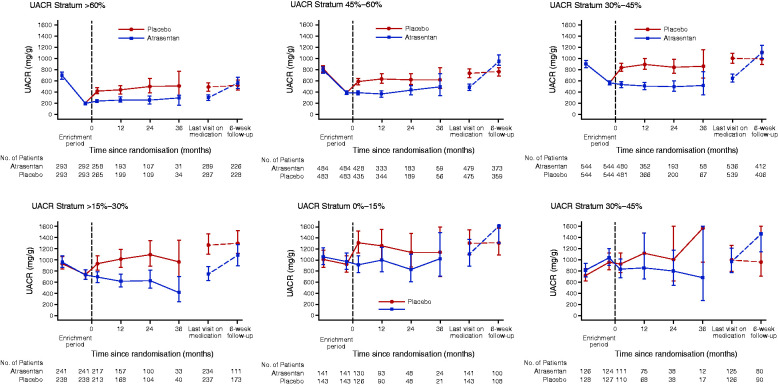
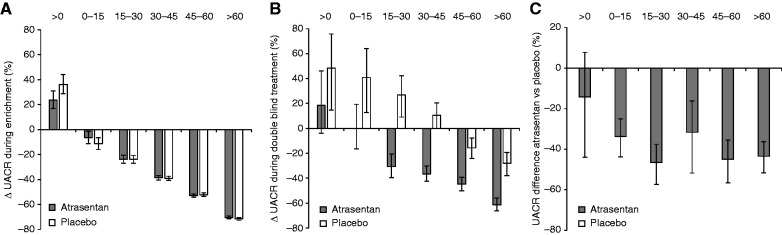
To examine why the association between UACR responses to atrasentan and kidney outcomes blunted after considering the placebo arm, we assessed UACR trajectories within the placebo group. Among participants who were transitioned to placebo at randomization, the change in UACR observed during the response-enrichment phase was similar compared with participants who continued atrasentan after randomization (Table 2, Figure 3 and 4A). However, during the double-blind treatment phase, UACR levels did not return to pre-enrichment values. Specifically, in the ≥60% UACR reduction and 45 to <60% UACR reduction strata, UACR levels remained below pre-enrichment values (Table 2, Figure 3 and 4B). In contrast, in the <15% UACR reduction and >0% UACR increase strata, UACR values increased to levels above pre-enrichment values (Table 2 and Figure 3). Thus, in the placebo group, where we expected that UACR would return to pre-enrichment values during double-blind treatment, we observed that the geometric mean change in UACR from pre-enrichment to the double-blind treatment phase decreased in the UACR responder strata, whereas it increased in UACR nonresponder strata (Table 2 and Figure 4B). Accordingly, the higher UACR response strata had lower UACR values and a lower incidence rate of the primary kidney outcome than the poor response strata (Supplemental Figure 3). The lower risk of the primary kidney outcomes persisted after adjustment for differences in patient characteristics (Figure 1B). Because the UACR values did not reverse to pre-enrichment values during double-blind treatment, the overall difference in UACR of 39.6% (95% CI, 35.0% to 43.8%) between atrasentan and placebo during double-blind treatment was consistent irrespective of the UACR response strata (P for heterogeneity 0.18; Figure 4C). Additional analyses also demonstrated no clear heterogeneity in placebo-subtracted effects of atrasentan on systolic BP across UACR response strata, although the largest UACR response stratum showed a greater BP difference (Supplemental Table 4).
Figure 3.
Changes in UACR during response enrichment and double-blind treatment in each UACR response stratum.
Figure 4.
Changes in UACR from (A) pre-enrichment to end-enrichment, and (B) pre-enrichment to double-blind treatment in the atrasentan and placebo group. (C) The difference in UACR between atrasentan and placebo during double-blind treatment.
UACR Changes During Wash-out
During the 6-week wash-out phase at the end of the trial, there was no change in UACR among placebo participants in any UACR response strata. Among atrasentan-assigned participants in the ≥60% UACR response stratum, the increase in UACR during wash-out was significantly less than the decrease during enrichment (P<0.001; Table 2 and Figure 3). In contrast, in the 15%–30% and <15% UACR response strata, the increase in UACR during wash-out was significantly larger than the reduction during enrichment (both P<0.001), whereas in the >0% UACR strata, UACR further increased after atrasentan discontinuation at the end of the trial (P<0.001; Figure 3).
Diuretic Treatment
Analyses to assess whether escalation of concomitant therapies during enrichment could have led to misclassification of the UACR response during the response enrichment phase showed that escalation of diuretics, defined as an increase of the dose or initiation of an additional diuretic, occurred in a greater proportion of patients in the higher UACR response strata (Table 3). Loop diuretics were most frequently escalated (Supplemental Table 5).
Table 3.
Number of patients (percentages) in whom diuretic treatment was escalated during enrichment in each UACR response stratum
| UACR Response Stratum | All Participants | Atrasentan | Placebo |
|---|---|---|---|
| (n=3668) | (n=1834) | (n=1834) | |
| ≥60% reduction | 156 (26.4) | 76 (25.6) | 80 (27.2) |
| 45%–60% reduction | 233 (24.1) | 116 (24.0) | 117 (24.2) |
| 30%–45% reduction | 207 (19.0) | 110 (20.2) | 97 (17.8) |
| 15%–30% reduction | 104 (21.7) | 53 (22.0) | 51 (21.4) |
| 0%–15% reduction | 59 (20.6) | 28 (19.7) | 31 (21.5) |
| ≥0% increase | 46 (18.1) | 14 (11.1) | 32 (25.0) |
| P value | 0.0039 | 0.019 | 0.033 |
Diuretic treatment could be initiated regardless whether patients were already using diuretics at start of enrichment. In combination with Table 1, the total number of patients using a diuretic can exceed 100%. Escalation of diuretic treatment was defined as an increase in the dose of the diuretic or initiation of an additional diuretic (e.g., initiation of a loop diuretic in a patient already using a thiazide diuretic).
Discussion
The design of the SONAR trial included an enrichment-response period, which enabled us to assess whether early changes in albuminuria predict long-term kidney outcomes in a placebo-controlled design. Within the atrasentan group, larger reductions in albuminuria were statistically significantly associated with a lower risk of kidney outcomes. In participants who transitioned to placebo treatment at randomization, we observed that the early reduction in albuminuria during response enrichment was associated with a lower risk of the kidney outcome. Accordingly, the predictive effect of UACR in the atrasentan group was eliminated after placebo correction. This resulted in a consistent relative risk reduction in kidney outcomes with atrasentan compared with placebo across all UACR strata. Because albuminuria levels after transitioning from atrasentan to placebo did not return to pre-enrichment values during double-blind treatment, the difference in UACR between atrasentan and placebo during double-blind treatment was also similar across all albuminuria response strata.
Earlier trials have shown that early reductions in albuminuria achieved with ACE inhibitors, ARBs, or sodium glucose cotransporter 2 inhibitors are significantly associated with long-term risk reductions for kidney end points.6,7,14,15 Furthermore, meta-analyses have demonstrated that early treatment effects on albuminuria of 25%–30% are strongly associated with significant benefits on clinical kidney end points.16 These data support a role for early change in albuminuria as a surrogate for clinical end points in CKD clinical trials. In accord with these studies, we observed within the atrasentan treatment group that a greater reduction in albuminuria in each responder stratum persisted during long-term atrasentan treatment, and was associated with a larger relative risk reduction for the primary kidney outcome. The current data are thus in accordance with those previously observed with ACE inhibitors or ARBs, but were achieved with an intervention that does not directly interfere in the renin-angiotensin system.
However, the previous post-hoc analysis are potentially biased because they correlate changes in albuminuria with outcomes, rather than compare outcomes between randomized groups. Therefore, the possibility that the lower risk of kidney outcomes across progressively higher albuminuria response strata was caused by factors unrelated to the albuminuria lowering effect of atrasentan cannot be excluded. We therefore adjusted the association between albuminuria changes and kidney outcomes for placebo effects. Within the placebo group, a larger reduction in albuminuria observed during atrasentan treatment in the enrichment period was, unexpectedly, associated with a lower incidence of kidney outcomes, so the predictive effect of early albuminuria changes during atrasentan was eliminated after placebo correction. This resulted in a consistent reduction in the risk of kidney outcomes with atrasentan compared with placebo across all UACR response strata, and consistent differences in albuminuria between atrasentan and placebo. Why was the association between albuminuria changes during enrichment and kidney outcomes observed in the atrasentan group eliminated after placebo correction? It is possible albuminuria change is not a causal predictor for the long-term effect of atrasentan and factors unrelated to the early albuminuria response to atrasentan explain the lower risk of kidney outcomes. However, it is also possible the early albuminuria change predicts the long-term benefit of atrasentan, but issues with respect to the imprecise assessment of albuminuria, regression-to-the-mean and introduction of concomitant medication during enrichment, did not enable us to ascertain this predictive effect.
To assess whether albuminuria responders comprised a selected subgroup with a genuinely lower risk of CKD progression, we compared the pretrial rate of eGFR decline in a random selection of SONAR participants. The similar rates of eGFR declines between albuminuria responders and nonresponders before response enrichment makes it less likely intrinsic differences in patient characteristics not accounted for in the analyses explain the observed association in the placebo group.
An alternative explanation for why we failed to identify atrasentan responders with optimal kidney protection compared with placebo may be attributed to methodological aspects relevant for future biomarker-based enrichment clinical trials. After randomization, the effect of atrasentan on UACR persisted during double-blind treatment in the atrasentan group, yet in the placebo group UACR levels did not return to baseline so differences in UACR between atrasentan and placebo were comparable during double-blind treatment in all response strata. These similar between-group differences in albuminuria would also explain the consistent long-term kidney protective effect of atrasentan. Why did albuminuria not return to baseline in the placebo group? There are several potential explanations for this unexpected finding. First, the observed albuminuria changes during the double-blind treatment phase could be affected by regression to the mean.17 This explanation is supported by the finding that the albuminuria levels in the placebo group in the highest response strata (>60% and 45%–60% UACR response) remained below pre-enrichment albuminuria values during double-blind treatment, whereas in the lowest UACR response strata (0%–15% and ≥0%) they exceeded pre-enrichment levels. This suggests the measured albuminuria values at baseline may be higher than the actual values in some responders, whereas in nonresponders the measured values may be lower than the actual values. This may have resulted in an overestimation or underestimation of the response in responders and nonresponders, respectively. An alternative explanation for why albuminuria did not return to baseline is that atrasentan may have longer-lasting effects than expected, and these persisted throughout the double-blind treatment phase in the placebo group. This legacy effect may explain the incomplete reversal and the lower than expected albuminuria levels in the placebo arm during double-blind treatment.
Second, albuminuria fluctuates within individuals over time. This complicates determination of the true change in albuminuria for an individual and thereby hampers separation between responders and nonresponders. In the SONAR trial, albuminuria varied substantially within an individual. The median within individual coefficient of variation in albuminuria during treatment with atrasentan was 26.6%. This magnitude is consistent with prior studies.18,19
A third explanation is that during the enrichment period, more patients in higher albuminuria response strata initiated diuretic treatment. Diuretic initiation was allowed per protocol to manage sodium and fluid retention. However, diuretics can also potentiate the effects of RAAS inhibition on BP and albuminuria lowering.20–22 Because all patients were using an inhibitor of the RAAS, the initiation of diuretic treatment added to RAAS inhibition may have potentiated the albuminuria lowering effect of RAAS inhibitors and obscured proper assessment of response to atrasentan.
The SONAR trial was terminated early by the sponsor because of a lower event rate than planned.12 This study shows albuminuria levels were lower than expected in patients randomized to placebo who responded to atrasentan during enrichment due to incomplete reversal of albuminuria after randomization. The lower-than-anticipated event rate may be attributable to the lower albuminuria levels because the average albuminuria levels during the double-blind treatment phase were associated with the primary kidney end point.
Four specific lessons learned from the SONAR trial will help inform design of future trials. First, when using an enrichment period to select trial participants, a wash-out period between the enrichment and randomization visit might minimize potential long-lasting effects and confirm reversibility of the initial drug response. Second, a crossover enrichment period can be considered to assess the individual drug response to a placebo response although this increases complexity and feasibility. Third, the magnitude of the within-individual variation in the biomarker to identify treatment responders should be carefully considered because a large variability hampers necessary precision to separate responders and nonresponders. Finally, initiation of concomitant medication should be avoided and carefully monitored to properly assess the individual response to the study medication.
This study has some limitations. First, SONAR was terminated early and was not designed or powered to assess the effect of atrasentan according the degree of albuminuria reduction during enrichment. However, the marked consistency in the effect of atrasentan compared with placebo on clinical end points makes it unlikely the results would be different if more end points were collected. Secondly, during follow-up, 20.7% of randomized participants discontinued study drug, which may have affected the estimation of the long-term efficacy of atrasentan. SONAR recruited patients with type 2 diabetes and CKD. All participants had severely increased albuminuria, which limits the generalizability of the results to the broader population of patients with type 2 diabetes and CKD.
In conclusion, the UACR change during open-label treatment with atrasentan predicted the primary kidney outcome. However, this association diminished after considering UACR changes during placebo treatment. These data indicate that, despite the careful assessment of albuminuria in the SONAR trial, we did not identify a greater kidney-protective effect of atrasentan compared with placebo in patients who experienced a larger albuminuria reduction during open-label treatment with atrasentan. Intrinsic patient factors associated with a lower risk of kidney failure, methodologic issues inherent to biomarker-based enrichment clinical trials, and aspects of the SONAR trial design and conduct may have contributed to the consistent effects of atrasentan compared with placebo on albuminuria and kidney outcomes during the double-blind treatment phase of the trial. Until we find a better way to randomize albuminuria responders to active and placebo treatment, the question remains whether the predictive performance of albuminuria response for kidney outcomes can be attributed to the albuminuria change alone.
Disclosures
D. de Zeeuw reports being the co-chair of the SONAR study steering committee; reports serving on advisory boards and/or speaker for Bayer, Boehringer Ingelheim, Fresenius, Mitsubishi-Tanabe, and Mundipharma; reports serving on the steering committees and/or as a speaker for AbbVie and Janssen; reports serving on the data safety and monitoring committees for Bayer; reports having consultancy agreements with AbbVie, Bayer, Boehringer Ingelheim, Fresenius, Janssen, Mitsubishi Tanabe, and Travere Pharmaceuticals; and reports receiving honoraria from Bayer, Boehringer Ingelheim, Fresenius, Janssen, Mitsubishi Tanabe, and Travere Pharmaceuticals. D. Kitzman reports being a member of the SONAR study steering committee and chair of the Event Adjudication Committee; reports receiving grant funding from AstraZeneca, Bayer, Novartis, Novo Nordisk, St. Luke's Hospital, and National Institutes of Health; reports being a consultant for AbbVie, AstraZeneca, Bayer, Merck, Boehringer Ingelheim, Corvia, CinRx, Duke Cardio Research Institute, GlaxoSmithKline (GSK), Novartis, Novo NorDisk, Pfizer, and St. Luke’s medical center; reports having an ownership interest in Gilead; reports receiving honoraria from AbbVie, AstraZeneca, Bayer, Boehringer Ingelheim, Duke Cardio Research Institute, CinRx, Corvia Medical, Novo Nordisk, Novartis, Merck, Pfizer, and St. Luke's Hospital; and reports being a scientific advisor or member of Bayer, Corvia Medical, and St. Luke's Hospital. D. Kohan reports being a member of the SONAR study steering committee; reports having consultancy agreements with AstraZeneca, Chinook, Janssen, and Travere; and reports being a scientific advisor or member of various journal editorial boards. D. Xie reports having other interests/relationships as International Society of Nephrology member. F.-F. Hou reports being a member of the SONAR study steering committee, a study investigator and a consultant for, and receiving honoraria from, AbbVie and AstraZeneca; and reports being a scientific advisor or member of the editorial board of Current Opinion Nephrology and Hypertension, Kidney Disease (Basel), Kidney International, and Kidney Medicine. G. Bakris was a member of the SONAR study steering committee and a study investigator; reports being on the steering committees of Canagliflozin and Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation, Candesartan and Lisinopril Microalbuminuria Study, and Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease and is principal investigator of Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease; reports being a consultant for Alnylam, AstraZeneca, Bayer, Boehringer Ingelheim, Cyclerion Therapeutics, Horizon Pharma, Ionis, Janssen, KBP Biosciences, Medscape, Merck, Novo Nordisk, Relypsa, Vascular Dynamics, and Vifor; reports receiving research funding from Bayer, Novo Nordisk, and Vascular Dynamics; reports receiving funding for steering committee activities and goes to the University of Chicago Medicine; reports receiving honoraria from Alnylam, AstraZeneca, Ionis, KBP Biosciences, Merck, Novo Nordisk, Teijin, and Vifor; reports being a scientific advisor or member of the American Heart Association, American Journal of Nephrology Editor, Diabetes Care Associate Editor, Hypertension Research Associate Editor, KBP Biosciences, Merck, Teijin, UpToDate Nephrology, and Vifor; and other interests/relationships with the American Diabetes Association, American Heart Association, Blood Pressure Council, and the National Kidney Foundation. H.-H. Parving reports being the co-chair of the SONAR study steering committee and serves as a consultant for AbbVie; reports having consultancy agreements with Abbott, AbbVie, AstraZeneca, and Novartis; reports having an ownership interest in Merck and Novo Nordisk; and reports receiving honoraria from Abbott, AbbVie, AstraZeneca, and Novartis. H. L. Heerspink reports being a member of the SONAR study steering committee and serves as a consultant for AbbVie, AstraZeneca, Bayer, Boehringer Ingelheim, Chinook, CSL Pharma, Dimerix, Gilead, GoldFinch, Janssen, Merck, Mundi Pharma, Mitsubishi Tanabe, Novo Nordisk, and Travere Pharmaceuticals; reports receiving research funding from AbbVie, AstraZeneca, Boehringer Ingelheim, and Janssen research support (grant funding directed to employer); and reports receiving Speakers Bureau from AstraZeneca. H. Makino reports being a member of the SONAR study steering committee and is a consultant for Boehringer Ingelheim, Teijin, and Travere Pharmaceuticals; and reports receiving speaker honoraria and being a scientific advisor or member of Boehringer Ingelheim. J. McMurray reports being a member of the SONAR study steering committee; reports receiving honoraria from personal lecture fees from Abbott Diabetes Care, Alkem Metabolics, Eris Lifesciences, Hikma, Lupin, Medscape/Heart.Org, ProAdWise Communications, Radcliffe Cardiology, Servier, Sun Pharmaceutical Industries Inc., and The Corpus; reports being a scientific advisor or member of Glasgow University for participation in advisory boards organized by AstraZeneca and Novartis; and reports having other interests/relationships via payments to employer Glasgow University for work on clinical trials, consulting, and other activities with AbbVie, Alnylam, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Cardurion, Cytokinetics, Dal-Cor, GSK, Ionis, KBP Biosciences, Novartis, Pfizer, and Theracos; and reports being a Director of Global Clinical Trial Partners Ltd. P. Rossing reports receiving honoraria to AstraZeneca, Boehringer Ingelheim, Novo Nordisk, and the Steno Diabetes Center Copenhagen from teaching, all to the institution; consultancy for Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Gilead, Novo Nordisk, Merck, Merck Sharp Dohme, Mundipharma, Sanofi, and Vifor; reports receiving research funding from AstraZeneca and Novo Nordisk; and reports being a scientific advisor or member of Astellas, AstraZeneca, Bayer, Gilead, Merck Sharp Dohme, Mundipharma, and Novo Nordisk, all honoraria to the institution. R. Correa-Rotter was a member of the SONAR study steering committee, serves on advisory boards for AstraZeneca and Boehringer; reports being a speaker for AbbVie, Amgen, AstraZeneca, Boehringer, Janssen, Sanofi, and Takeda; reports having consultancy agreements with AstraZeneca, Boheringer Ingelheim, GSK, Medtronic, and Novo Nordisk; reports receiving research funding from AstraZeneca, GSK, and Novo Nordisk; reports receiving honoraria from Amgen, AstraZeneca, Boehringer Ingelheim, Janssen, and Takeda (honoraria for consultancy or speaker); reports being a scientific advisor or member as Associate Editor of Blood Purification, AstraZeneca, GSK, Editorial Board Current Opinions Nephrology and Hypertension, National Leader FLOW study, Nefrologia Latinoamericana, Novo Nordisk, Revista de Investigación Clinica, and the Steering Committee of Dapagliflozin and Prevention of Adverse Outcomes in CKD, National Leader of the ASCEND study; and other interests/relationships as a Member of European Renal Association, the International Society of Nephrology, Latin American Society of Nephrology and Hypertension, Mexican Institute for Research in Nephrology, and the National Kidney Foundation. V. Perkovic reports being a member of the SONAR study steering committee; reports serving on Steering Committees for trials funded by AbbVie, Boehringer Ingelheim, GSK, Janssen, Novo Nordisk, Retrophin, and Tricida; reports participating in Scientific Presentations/Advisory boards with AbbVie, Astellas, AstraZeneca, Bayer, Baxter, Bristol Myers Squibb, Boehringer Ingelheim, Dimerix, Durect, Eli Lilly, Gilead, GSK, Janssen, Merck, Mitsubishi Tanabe, Novartis, Novo Nordisk, Pfizer, Pharmalink, Relypsa, Retrophin, Sanofi, Servier, and Tricida; reports having consultancy agreements with AbbVie, Astellas, AstraZeneca, Bayer, Baxter, Bristol-Myers Squibb, Boehringer Ingelheim, Dimerix, Durect, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Merck, Metavant, Mitsubishi Tanabe, Mundipharma, Novartis, Novo Nordisk, Pfizer, PharmaLink, Relypsa, Retrophin, Roche, Sanofi, Servier, Tricida, UptoDate, and Vitae; and reports receiving research funding from the Australian National Health and Medical Research Council (Senior Research Fellowship and Program Grant).
Funding
The SONAR trial was funded by AbbVie.
Supplementary Material
Acknowledgments
G. Bakris, R. Correa-Rotter, D. de Zeeuw, H. Heerspink, F. Hou, D. Kitzman, D. Kohan, H. Makino, J. McMurray, H.-H. Parving, V. Perkovic, and P. Rossing designed the study; H. Heerspink and D. Xie analyzed the data; H. Heerspink and D. de Zeeuw wrote the first draft of the article; all authors were involved in collecting the data, data interpretation, drafting and critically revising the article, and reviewed and approved the final manuscript. All authors had access to study results, and the lead author takes responsibility for the integrity of the data and accuracy of the data reported. The authors thank all investigators, trial teams, and patients for their participation in the trial.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial “Predictive Enrichment in Kidney RCTs: Is Albuminuria the Answer?,” on pages 2689–2691.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021030391/-/DCSupplemental.
Supplemental Table 1. Baseline characteristics according predefined UACR response strata.
Supplemental Table 2. Baseline characteristics of participants with available eGFR data before enrollment in the SONAR trial.
Supplemental Table 3. Changes in risk markers of cardiovascular and kidney disease progression in predefined UACR response strata during the response-enrichment period.
Supplemental Table 4. Effects of atrasentan compared with placebo on systolic BP during double-blind treatment in UACR response strata.
Supplemental Table 5. Number of patients (percentages) in whom thiazide, loop, and potassium sparing diuretic treatment was escalated during enrichment in each UACR response stratum.
Supplemental Figure 1. Effect of atrasentan compared with placebo on rate of eGFR decline during the double-blind treatment period according to UACR response during the response enrichment period.
Supplemental Figure 2. Effect of atrasentan compared with placebo on hospitalizations for heart failure during the double-blind treatment period according to UACR response during the response-enrichment period.
Supplemental Figure 3. Association between the geometric mean UACR level achieved during double-blind treatment and event rate for the primary kidney outcome according to the UACR response recorded during the response-enrichment period.
Supplemental Summary 1. Full member list of the SONAR investigators.
References
- 1.Webster AC, Nagler EV, Morton RL, Masson P: Chronic kidney disease. Lancet 389: 1238–1252, 2017 [DOI] [PubMed] [Google Scholar]
- 2.Levin A, Tonelli M, Bonventre J, Coresh J, Donner JA, Fogo AB, et al. ; ISN Global Kidney Health Summit participants : Global kidney health 2017 and beyond: A roadmap for closing gaps in care, research, and policy. Lancet 390: 1888–1917, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Andrassy KM: Comments on ‘KDIGO clinical practice guideline for the evaluation and management of chronic kidney disease’. Kidney Int 84: 622–623, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. ; DAPA-CKD Trial Committees and Investigators : Dapagliflozin in patients with chronic kidney disease. N Engl J Med 383: 1436–1446, 2020 [DOI] [PubMed] [Google Scholar]
- 5.Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, et al. ; FIDELIO-DKD Investigators : Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 383: 2219–2229, 2020 [DOI] [PubMed] [Google Scholar]
- 6.Heerspink HJL, Ninomiya T, Persson F, Brenner BM, Brunel P, Chaturvedi N, et al. : Is a reduction in albuminuria associated with renal and cardiovascular protection? A post hoc analysis of the ALTITUDE trial. Diabetes Obes Metab 18: 169–177, 2016 [DOI] [PubMed] [Google Scholar]
- 7.Oshima M, Neuen BL, Li J, Perkovic V, Charytan DM, de Zeeuw D, et al. : Early change in albuminuria with canagliflozin predicts kidney and cardiovascular outcomes: A post hoc analysis from the CREDENCE trial. J Am Soc Nephrol 31: 2925–2936, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waijer SW, Xie D, Inzucchi SE, Zinman B, Koitka-Weber A, Mattheus M, et al. : Short-term changes in albuminuria and risk of cardiovascular and renal outcomes in type 2 diabetes mellitus: A post hoc analysis of the EMPA-REG OUTCOME Rrial. J Am Heart Assoc 9: e016976, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossing P, Hommel E, Smidt UM, Parving HH: Reduction in albuminuria predicts a beneficial effect on diminishing the progression of human diabetic nephropathy during antihypertensive treatment. Diabetologia 37: 511–516, 1994 [DOI] [PubMed] [Google Scholar]
- 10.Roscioni SS, Lambers Heerspink HJ, de Zeeuw D: Microalbuminuria: Target for renoprotective therapy PRO. Kidney Int 86: 40–49, 2014 [DOI] [PubMed] [Google Scholar]
- 11.de Zeeuw D, Coll B, Andress D, Brennan JJ, Tang H, Houser M, et al. : The endothelin antagonist atrasentan lowers residual albuminuria in patients with type 2 diabetic nephropathy. J Am Soc Nephrol 25: 1083–1093, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heerspink HJL, Parving H-H, Andress DL, Bakris G, Correa-Rotter R, Hou F-F, et al. ; SONAR Committees and Investigators : Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): A double-blind, randomised, placebo-controlled trial. Lancet 393: 1937–1947, 2019 [DOI] [PubMed] [Google Scholar]
- 13.Heerspink HJL, Andress DL, Bakris G, Brennan JJ, Correa-Rotter R, Dey J, et al. : Rationale and protocol of the Study Of Diabetic Nephropathy with AtRasentan (SONAR) trial: A clinical trial design novel to diabetic nephropathy. Diabetes Obes Metab 20: 1369–1376, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, et al. : Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: Lessons from RENAAL. Kidney Int 65: 2309–2320, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Coresh J, Heerspink HJL, Sang Y, Matsushita K, Arnlov J, Astor BC, et al. ; Chronic Kidney Disease Prognosis Consortium and Chronic Kidney Disease Epidemiology Collaboration : Change in albuminuria and subsequent risk of end-stage kidney disease: An individual participant-level consortium meta-analysis of observational studies. Lancet Diabetes Endocrinol 7: 115–127, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heerspink HJL, Greene T, Tighiouart H, Gansevoort RT, Coresh J, Simon AL, et al. ; Chronic Kidney Disease Epidemiology Collaboration : Change in albuminuria as a surrogate endpoint for progression of kidney disease: A meta-analysis of treatment effects in randomised clinical trials. Lancet Diabetes Endocrinol 7: 128–139, 2019 [DOI] [PubMed] [Google Scholar]
- 17.Bland JM, Altman DG: Regression towards the mean. BMJ 308: 1499, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Witte EC, Lambers Heerspink HJ, de Zeeuw D, Bakker SJ, de Jong PE, Gansevoort R: First morning voids are more reliable than spot urine samples to assess microalbuminuria. J Am Soc Nephrol 20: 436–443, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrykiv SI, de Zeeuw D, Persson F, Rossing P, Gansevoort RT, Laverman GD, et al. : Variability in response to albuminuria-lowering drugs: True or random? Br J Clin Pharmacol 83: 1197–1204, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vogt L, Waanders F, Boomsma F, de Zeeuw D, Navis G: Effects of dietary sodium and hydrochlorothiazide on the antiproteinuric efficacy of losartan. J Am Soc Nephrol 19: 999–1007, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esnault VL, Ekhlas A, Delcroix C, Moutel MG, Nguyen JM: Diuretic and enhanced sodium restriction results in improved antiproteinuric response to RAS blocking agents. J Am Soc Nephrol 16: 474–481, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Kwakernaak AJ, Krikken JA, Binnenmars SH, Visser FW, Hemmelder MH, Woittiez AJ, et al. ; Holland Nephrology Study (HONEST) Group : Effects of sodium restriction and hydrochlorothiazide on RAAS blockade efficacy in diabetic nephropathy: A randomised clinical trial. Lancet Diabetes Endocrinol 2: 385–395, 2014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.