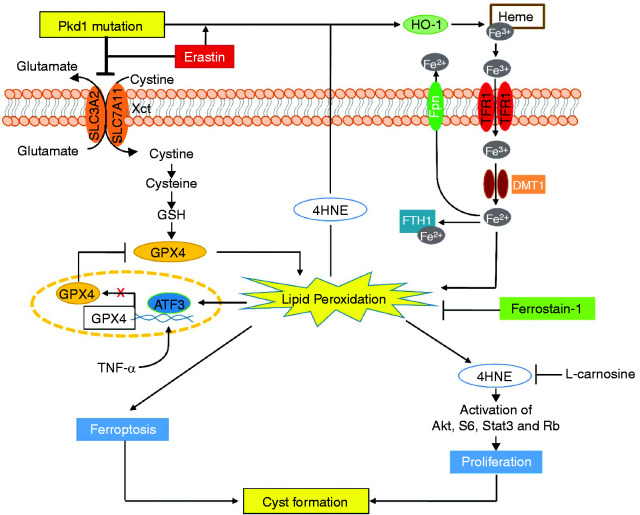
Figure 8.
Working model of ferroptosis in regulation of cyst growth in ADPKD. A schematic diagram depicting ferroptosis associated pathways and processes in Pkd1 mutant renal epithelial cells and kidneys. Pkd1 mutation or erastin treatment (1) decreased system Xc− and resulted in the reduction of cystine uptake and the depletion of GSH, leading to inactivate GPX4 enzymatic activity and then increase lipid peroxidation, and (2) increased the expression of multiple iron regulatory proteins (IRPs), including TFR1, FTH1, DMT1, Fpn, and HO-1 and resulted in the increase of the import of HO-1–mediated nonheme iron and the storage of iron in Pkd1 mutant renal epithelial cells to increase lipid peroxidation, both of these processes leading to ferroptosis. The excess lipid peroxidation also stimulated the expression of ATF3, which repressed the expression of SLC7A11 and GPX4, forming a feedback loop of lipid peroxidation–ATF3–SLC7A11/GPX4–lipid peroxidation, and increased the production of 4HNE-protein adducts. Upregulation of 4HNE (1) promotes Pkd1 mutant cell proliferation via activation of Akt, S6, Stat3, and Rb, and (2) upregulates the expression of HO-1, which regulates the release of heme iron and results in its import and storage in Pkd1 mutant renal epithelial cells to promote ferroptosis. All these 4HNE-mediated processes can be inhibited by L-carnosine. In addition, treatment with Fer-1, a ferroptosis inhibitor, delays cyst growth in early stage and long-lasting Pkd1 animal models, which highlights a novel therapeutic strategy for ADPKD treatment.